Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Case Report - (2024)Volume 15, Issue 1
Arrhythmogenic right ventricular dysplasia is a cardiomyopathy of genetic origin characterized by predominant right ventricular involvement, episodes of ventricular arrhythmias with an increased risk of sudden death.
We report the case of a 20-year-old asymptomatic professional soldier who consulted for an annual check-up. The electrocardiogram revealed an abrasion of the anteroseptal R wave, persistent S wave in V5 and V6, an S1S2S3 pattern and negative T waves in the anterior and inferior leads. The echocardiogram and cardiac Magnetic Resonance Imaging (MRI) revealed major criteria (RV dilatation with end diastolic volume 147 ml, global RV hypokinesia and dyskinesia located at the basal and apical inferoseptal wall) confirming the diagnosis of arrhythmogenic right ventricular dysplasia. Also, dense fibrosis was seen in the interventricular septum and the basal anterior and lateral walls of the Left Ventricle (LV) suggesting biventricular involvement.
The patient remained asymptomatic after one year without treatment. This clinical case highlights the importance of imaging in the diagnosis of rare cardiovascular diseases such as biventricular arrhythmogenic dysplasia.
Biventricular arrhythmogenic dysplasia; Young; Senegal; Echocardiogram; Left ventricle
Arrhythmogenic cardiomyopathies are a heterogeneous group of cardiac muscle anomalies of diverse origins, characterized by the occurrence of severe ventricular arrhythmias without ischemic, valvular or hypertensive causes. Arrhythmogenic Right Ventricular Dysplasia (ARVD), of genetic origin, is the best described of the arrhythmogenic cardiomyopathies. It is most often linked to desmosomal abnormalities that are pathophysiologically responsible for fibroadiputic remodeling of the right ventricular myocardium [1].
Initially regarded as a Right Ventricular (RV) disease, ARVD has turned out to be a polymorphous pathology, with the existence of biventricular and left-predominant forms, described in particular with the advent of cardiac Magnetic Resonance Imaging (MRI) [1,2].
We report a particularly rare case of a biventricular form of ARVD. This case highlights the importance of imaging in the diagnosis of rare cardiovascular pathologies at risk of sudden death.
This was a 20-year-old asymptomatic black man from the military function, who came to the cardiology department for an annual check-up.
At the physical examination, the patient was apyretic, with a heart rate of 62 beats per minute (bpm). Blood pressure was normal. Cardiovascular examination was normal.
The electrocardiogram showed a regular sinus rhythm at 62 bpm, a fixed PR of 160 ms, an abrasion of the anteroseptal R wave, an S1S2S3 aspect and repolarization disorders with negative T waves on anterior and inferior leads. Bazett-corrected QT was normal at 410 ms (Figure 1).
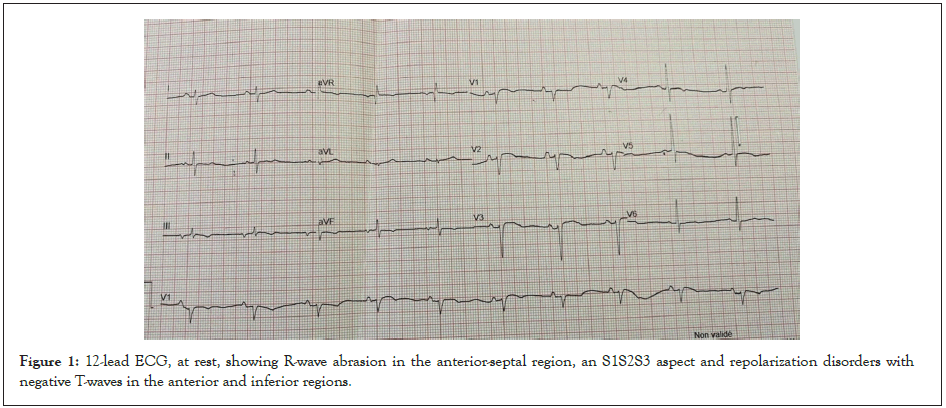
Figure 1: 12-lead ECG, at rest, showing R-wave abrasion in the anterior-septal region, an S1S2S3 aspect and repolarization disorders with negative T-waves in the anterior and inferior regions.
Transthoracic echocardiography revealed akinesia with right midventricular dilatation (Figure 2), a blistered aspect of the right ventricular free wall with moderate impairment of its longitudinal systolic function (TAPSE: 14 mm, tricuspid S wave: 6.4 cm/s) . The RV outflow chamber was measured at 38 cm at left para sternal short axis and its shortening fraction at 11% (Figures 2A-2D).
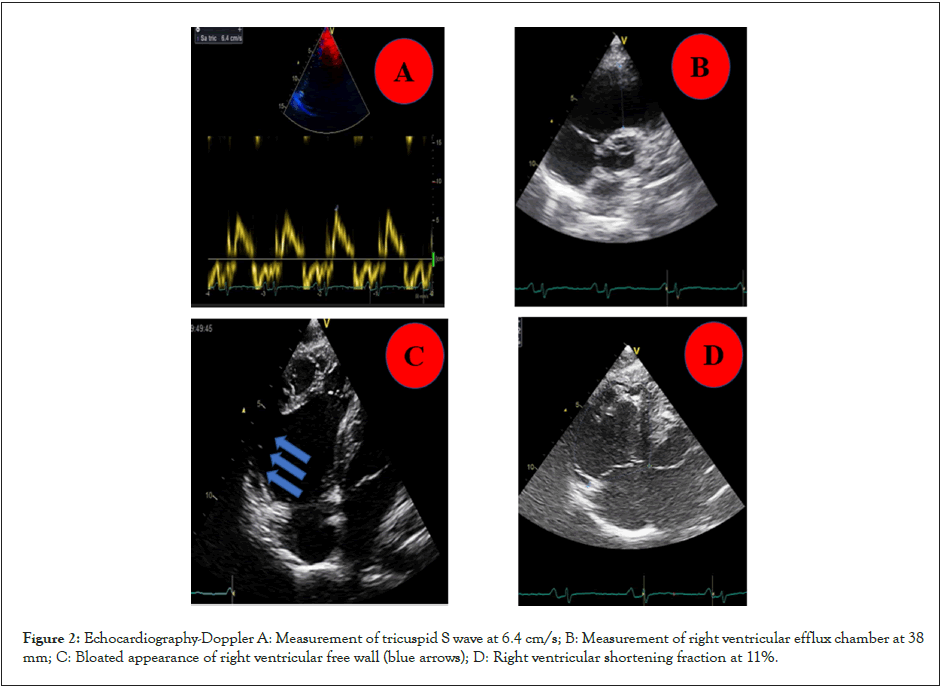
Figure 2: Echocardiography-Doppler A: Measurement of tricuspid S wave at 6.4 cm/s; B: Measurement of right ventricular efflux chamber at 38 mm; C: Bloated appearance of right ventricular free wall (blue arrows); D: Right ventricular shortening fraction at 11%.
The right atrium was moderately dilated. Systolic pulmonary artery pressure was estimated at 28 mmHg. There was a discrete septal hypokinesia with a moderately impaired left ventricular ejection fraction of 43% by Simpson Biplan (Figure 3). The inferior vena cava was thin and compliant. The pericardium was normal as well as the initial part of the aorta and pulmonary artery.
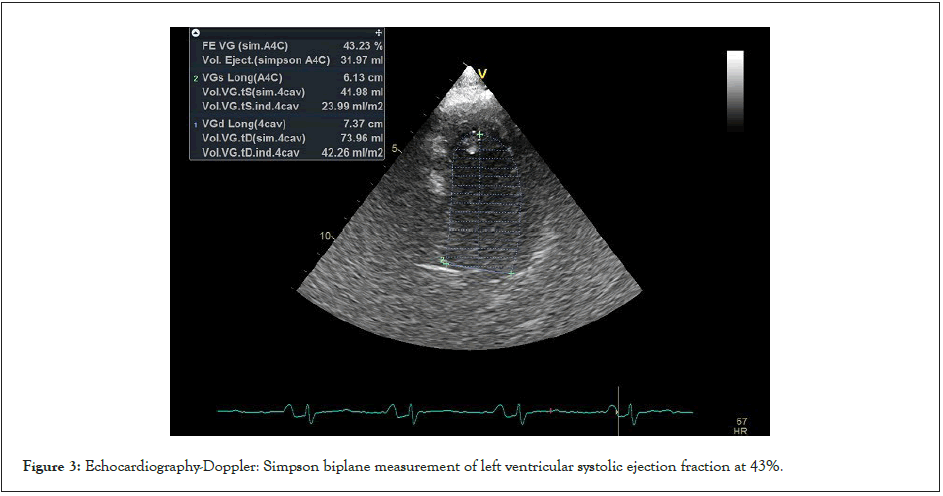
Figure 3: Echocardiography-Doppler: Simpson biplane measurement of left ventricular systolic ejection fraction at 43%.
Cardiac MRI showed a right ventricular dilatation (telediastolic volume 147 ml), a global RV hypokinesia and a dyskinesia localized to the basal and apical inferoseptal wall (Figure 4). Dense fibrosis was associated with the interventricular septum and the basal anterior and lateral walls of the LV (Figure 5). This suggested bi-ventricular arrhythmogenic dysplasia. The LV septal wall was hypokinetic and thick, measuring 14 mm in diastole, and left ventricular function was at 42%. The right ventricular function was severely impaired, measured at 13%.
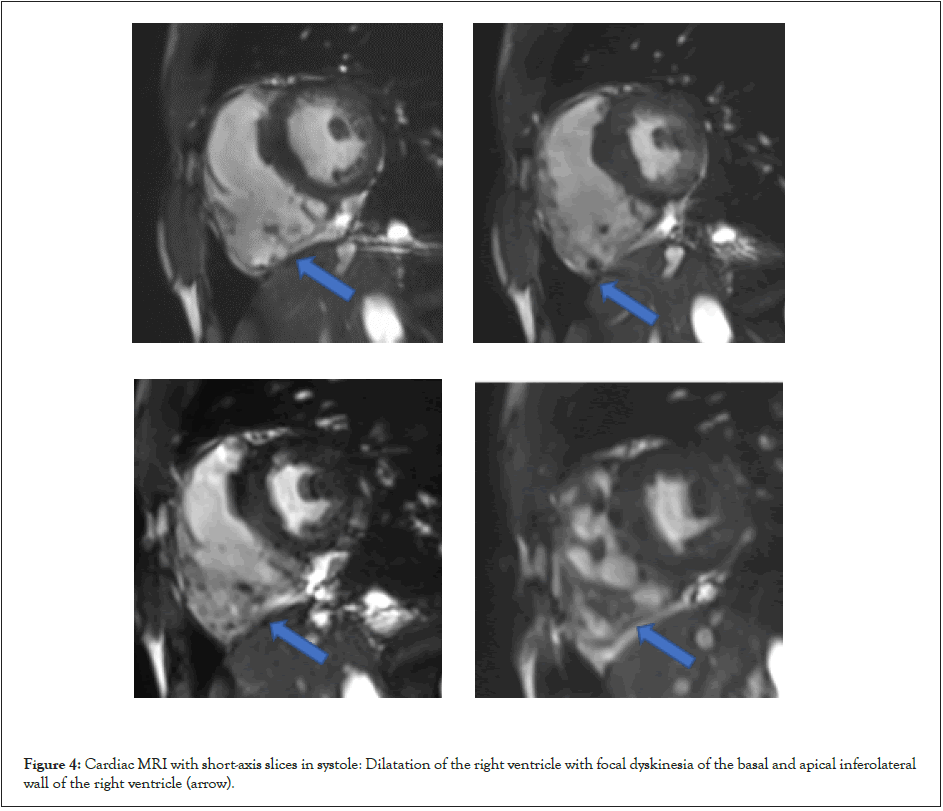
Figure 4: Cardiac MRI with short-axis slices in systole: Dilatation of the right ventricle with focal dyskinesia of the basal and apical inferolateral wall of the right ventricle (arrow).
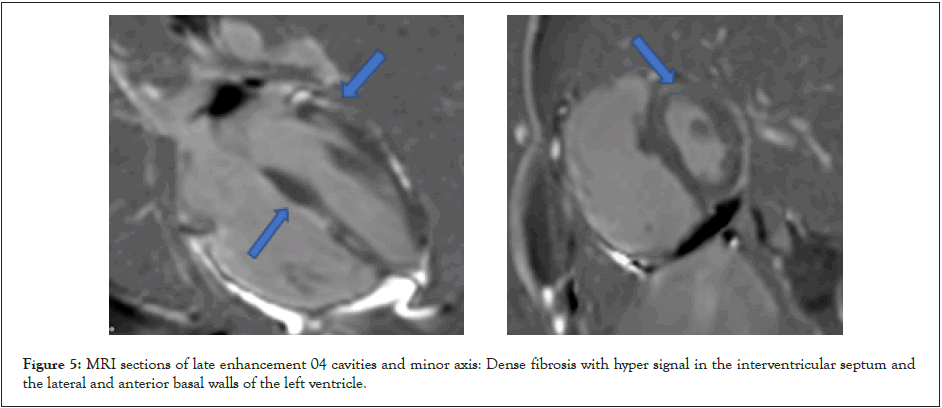
Figure 5: MRI sections of late enhancement 04 cavities and minor axis: Dense fibrosis with hyper signal in the interventricular septum and the lateral and anterior basal walls of the left ventricle.
This was consistent with biventricular arrhythmogenic dysplasia. Rhythmic holter and high-amplification ECG, sometimes useful in the management, were not performed in our patient.
ARVD is a rare condition with an estimated global prevalence of 1:5000 [3]. It was first described by Fontaine in 1972. Fibrofatty remodeling preferentially affects the RV, creating re-entry circuits and impairing the right ventricular function [3]. Few cases have been reported in sub-Saharan Africa [4]. The South African ARVD registry found 50 cases of ARVD over a 5-year period, with a male predominance (66%) and an average age of 32 years at diagnosis. Clinical presentation was dominated by palpitations, dizziness and chest pain. Only one patient (2%) was asymptomatic [5] as in our case. In an American series of 100 patients, palpitations (27%), syncope (26%) and dizziness (14%) were the main symptoms [6]. Fifteen percent of patients were asymptomatic, while 23% had sudden death as their first manifestation, diagnosed at autopsy [6]. The high prevalence of asymptomatic forms and/or forms revealed by sudden cardiac death reflects the severity of the disease.
The particularity of our observation is the fortuitous discovery of a biventricular form in an asymptomatic patient.
The diagnosis of ARVD is based on criteria established by the task force of the European Society of Cardiology (ESC) [7]. In our context, it is hampered by the limited availability of human resources and paraclinical examinations such as echocardiography, cardiac MRI and genetic testing. The latter, when they exist, are not easily accessible due to their high cost in relation to the population's incomes.
However, the electrocardiogram is a key diagnostic test for patients with suspected ARVD. Among the most frequent signs are anteroseptal T-wave negativation (76%) and the presence of an epsilon wave (56%) [5]. Lateral T-wave negativation, observed in our patient, points to left ventricular involvement [3].
Transthoracic Echocardiography (TTE) is available in all regions of Senegal and is relatively accessible. Since cardiac MRI is not widely available, TTE is of vital importance in our context. In certain situations, in association with the ECG, it can lead to the diagnosis of ARVD. In our patient, major signs of ARVD were identified. In forms with biventricular involvement, echocardiography may be inconclusive, raising the possibility of other dilated cardiomyopathies [3,7]. Cardiac MRI may help rectify the diagnosis, by demonstrating the major criteria noted in our patient.
In the South African series, no biventricular forms were documented [5]. In the American series, 1 patient developed a biventricular form [6].
All this explains the important role played by investigations such as echocardiography and cardiac MRI, which in our countries enable the diagnosis of rare and potentially dangerous pathologies such as ARVD.
ARVD is associated with various genetic anomalies, most often autosomal dominant [8,9]. Genetic tests, which are not available in our region, could have helped identify the gene(s) involved and facilitated screening of our patient's first-degree relatives.
However, the prognosis of ARVD depends largely on the rhythmic risk and the etiological investigation. The holter- Electrocardiography (ECG) could not be performed in our patient because of his refusal to continue the treatment. Investigation for sudden cardiac death in the family, history of cardiac arrest due to ventricular fibrillation or Ventricular Tachycardia (VT), and rhythmic syncope was negative. Nevertheless, the biventricular systolic dysfunction noted in this young serviceman is a powerful risk marker and is important for the management [10,11].
Management is multi-faceted. It includes prevention of sudden cardiac death from ventricular arrhythmia, treatment of heart failure and heart transplantation. By assessing the risk of sudden cardiac death, and after discussion with the patient, an Implantable Cardioverter Defibrillator (ICD) can be indicated for primary or secondary prevention. ICDs are the only effective preventive treatment against sudden cardiac death. The survival benefit is estimated at 26% and it is most often a single-chamber ICD [3].
Beta-blockers, indicated in all patients with an ARVD, are the therapy of choice for controlling rhythmic risk [12]. Where beta- blockers are ineffective, the use of other antiarrhythmic drugs such as sotalol, amiodarone or flecaine has been described. The efficacy of these molecules in this indication remains controversial [3].
Apart from a formal ban on sporting activities, our patient did not receive any treatment because he did not approve of it. However, he remains asymptomatic after one year without drug treatment.
ARVD is a rare and under-diagnosed form of cardiomyopathy in sub-Saharan Africa. Its prognosis is essentially linked to the risk of sudden cardiac death due to ventricular rhythm disorders. Biventricular involvement is a poor prognosis factor. Better access to various cardiovascular imaging modalities and local expertise in genetic testing are needed to assess its true prevalence and improve its diagnosis and management.
I have obtained written informed consent from the patient(s) for the publication of this case report accompanying data and images.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mboup WN, Tidiane NSC, Moustapha D, Khadidiatou D, Madjiguène KM, Rabab Y, et al. (2024) Arrhythmogenic Right Ventricular Dysplasia: A Particularly Rare Case of Biventricular Involvement in Senegal. J Clin Exp Cardiolog.15:867.
Received: 01-Dec-2023, Manuscript No. JCEC-23-28269; Editor assigned: 04-Dec-2023, Pre QC No. JCEC-23-28269 (PQ); Reviewed: 18-Dec-2023, QC No. JCEC-23-28269; Revised: 25-Dec-2023, Manuscript No. JCEC-23-28269 (R); Published: 02-Jan-2024 , DOI: 10.35248/2155-9880.24.15.867
Copyright: © 2024 Mboup WN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.