Journal of Horticulture
Open Access
ISSN: 2376-0354
ISSN: 2376-0354
Research Article - (2015) Volume 2, Issue 4
Citrus production in Ethiopia is threatened by a number of biotic and abiotic factors. Among these citrus gummosis is one of the most important biotic constraints in the country. This study was conducted with the objective to measure and estimate the incidence and distribution of citrus gummosis (Phytophthora spp.) in major citrus growing areas of Ethiopia. Surveys were conducted in ten major citrus growing areas of Ethiopia. In each orchard, 30 trees were selected and totally 300 trees were assessed for gummosis incidence. These trees were sampled by making two diagonal transects across the field in the form of an “X”. A tree decline was scored on a 0-4 scale where 0=free of any decline symptom, 4=complete drying of the plant. A tree was defined and recorded as cankered when it had any of the following symptoms: discoloration of the bark surface, discoloration of the underlying tissues, dieback, dried the whole part of the plant and exudation of gum from infected tissues. Results showed that growth and fruit production are greatly reduced on trees infected by this disease. Cracked lesions that exude sap are found on infected scions, which become gradually girdled and killed. This symptom was prevailed in 90% of the surveyed citrus orchards. Gummosis was more frequent on scions (trunk and branch) which had 66.96% infection than rootstocks (33.02%). generally the issue needs more attention in the field management including harvesting method (avoid climbing on the tree rather through ladder), don’t touch the tree with the equipments and hands with mud probabily have an inoculums; when irrigating and weeding and finally use of fungicides when pruning of the diseased branch.
<Keywords: Citrus; Assessment; Dieback; Gummosis; Phytophthora; Survey; Ethiopia
Citrus (Citrus sinesis L.) is one of the most important fruit crops known by humans since ancient times and is a good source of vitamin C with high antioxidant potential [1]. The origin of citrus is believed to be south eastern Asia including eastern Arabia to the Philippines and from the Himalayas south to Indonesia or Australia [2]. Citrus constitutes a major group of fruits comprising of mandarins, oranges, lemon, pummelo, grape fruits, tangelo, trifoliate orange, citron, and citranges [3]. Citrus is among the most important fruit crops in Ethiopia. Its cultivation started in Upper Awash valley and Melkassa areas in central Ethiopia [4]. It is being produced mainly in Dire Dawa areas, lower and middle Awash and Melkassa areas in southeast region of Ethiopia. Ethiopia's agro-climatic conditions are highly suitable for production of high quality citrus fruits. Five phenological regions were identified based on the blooming season and climate as potential production centers capable of supplying citrus throughout the year [5]. It occupied 7290 hectares of land in 1985 in the country, but the area has come down to 5,380 hectares (2200 oranges, 1750 mandarin and 1100 hectares limes and lemon) with a production of 33,500 metric tons only [6]. The decline in production land through time was attributed to a number of production limiting biotic and abiotic factors.
Hence, there is a need to undertake coordinated and multi-phased research investigations on possible causes of citrus decline and find a sustainable, eco-friendly and easily commercialized technology that fits for our subsistence agriculture system. Determining the actual importance of a possible citrus decline factors viz gummosis (Phytophthora spp. ) with the aim to develop eco-friendly and sustainable measures for their management is an integral part of this coordinated research effort. Foot rot and gummosis occur when Phytophthora propagules are splashed onto the trunk near ground level, infect wounds or growth cracks and produce lesions which extend down to the bud union and cause a twig dieback and if severe, trees may eventually die [7]. Few studies have suggested a possible mechanism of gummosis occurrence on citrus tree and as a cause of for citrus decline elsewhere in the world much work was not done in Ethiopia thus far on gummosis. Consequently this study was conducted with the objective to measure and estimate the incidence and distribution of citrus gummosis (Phytophthora spp.) from major citrus growing areas of Ethiopia.
Description of the study area
A survey was conducted between October and November 2011 to determine the incidence and distribution of gummosis (Phytophthora spp.) in major citrus growing areas of Ethiopia. In all orchards, the root stock was sour orange and the scion was the sweet orange cultivar Valencia in all the surveyed orchards. Disease survey was conducted in ten established citrus orchards in Upper awash, Errer-Gota, and Koka areas. The sites were located at 9°54’ N, 37°43’ E and 1175 masl (upper awash), 10°54’N, 37°75’E and 1174 masl (Erer Gota) and 9°28’N, 37°50’E and 1611masl (Koka) areas. The average minimum and maximum annual temperature were 15.3°C and 32.6°C at Upper Awash, 17°C and 29°C Erer-Gota.
Disease assessment
In each orchard, 30 trees were selected and a gummosis incidence was assessed. These trees were sampled by making two diagonal transects across the field in the form of an “X” (15 plants along each diagonal). Gummosis incidence was determined as the proportion of plants showing gummosis symptoms, expressed as a percentage of the total number of plants assessed [8]. A tree was defined and recorded as cankered when it had any of the following symptoms: discoloration of the bark surface, discoloration of the underlying tissues, dieback, dried the whole part of the plant and exudation of gum from infected tissues. Tree decline was scored on a 0-4 scale where 0=free of any decline symptom, 4= complete drying of the plant (Table 1). Disease severity index (DSI) was then calculated as:
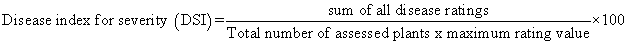
| Disease scale | Description of disease status |
|---|---|
| 0 | Tree with no symptom associated with gummosis |
| 1 | Decline symptom associated with gummosis up to 25% of the branch affected |
| 2 | widespread decline of the branch associated with gummosis up to 25-50% of the branch affected |
| 3 | Decline and death of the branch associated with gummosis up to 50-75% of the branch affected |
| 4 | Decline of 75-100% tree, including dead tree. |
Table1: Category of decline of citrus trees based on Asad, et al.[9].
Disease scale Description of disease status 0Tree with no symptom associated with gummosis1Decline symptom associated with gummosis up to 25% of the branch affected 2widespread decline of the branch associated with gummosis up to 25-50% of the branch affected 3Decline and death of the branch associated with gummosis up to 50-75% of the branch affected4Decline of 75-100% tree, including dead tree.
Isolation of Phytophthora spp. from bark
Samples with underlying tissue were excised using a sharp knife which was surface disinfected through wiping with ethyl alcohol before and after each use. The disease samples were taken from the active lesion near to the crown and branch showing the symptom of the disease. A total of 30 samples, obtained from 10 surveyed orchards, were processed. Pieces of 3-5 mm-long tissue were transferred using sterile forceps to a Petri dish containing modified V-8 agar medium and incubated at 23°C in the dark and examined within 3–5 days. The modified V-8 agar consisted of eight vegetable agar (V-8 agar) composed of 2 g CaCO3, 200 mL V8 juice and 15 g agar in 800 mL distilled water amended with 20 mg Pimaricin, 150 mg Ampicilin, 75 mg Vancomycin, 50 mg Dichloran, which were dissolved in 10 ml distilled sterile water and added to 1 liter of V-8 agar [10]. Pure cultures of Phytophthora spp. were obtained by transferring hyphal tips onto potato dextrose agar (PDA) which was used for colony pattern description, while V8 juice agar was used for morphological description.
Distribution of citrus gummosis (phytophthora spp.)
The survey results have shown that the disease was prevalent in all the surveyed orchards with different magnitude of infection. The gumming and cracking symptoms were observed on root stock, trunks and branches which produced a light yellow and brown gum across the sampling orchards. More than 70% of surveyed orchards the dieback symptoms of the tree were observed along with the gumming from main branch (Figure 1). Branches with this symptom resulted in die back of a tree that could not keep their fruits and leaves alive for the next season. Alvarez et al. (2007) the disease Phytophthora gummosis causes necrosis of the inner bark and the cambium of the trunk and Phytophthora root rot, foot rot also may cause tree decline in severe cases tree wilt and death.
Erer and Hurso the disease incidence reaches up to 100% while at Tibilla 0.0% were recorded at the time of survey (Table 2). In the root stock the highest gummosis frequency were recorded at Erer (70%) followed by Hurso (46.67%). However, at the trunk as well as the branch the highest frequency were recorded at Hurso with a 90% and 63.33% followed by Erer 83.33%, and 53.33% frequency respectively.
| No | Locations | Orchardin | Sample size | Incidence (%) | Tree decline a |
|---|---|---|---|---|---|
| 1 | Dire Dawa | Tony farm | 30 | 60 | 1.6 |
| 2 | Hurso | Hurso | 30 | 100 | 2.875 |
| 3 | ErrerGota | Erer | 30 | 100 | 3.642 |
| Gota | 30 | 26.7 | 2.375 | ||
| Fetule | 30 | 86.7 | 2.625 | ||
| 4 | Upper Awash | Nura Era | 30 | 13.3 | 2.092 |
| Merti | 30 | 16.7 | 1.53 | ||
| Tibilla | 30 | 0 | 0.917 | ||
| 5 | Melkassa | Melkassa | 30 | 33.3 | 1.167 |
| 6 | Koka | Koka area | 30 | 13.3 | 2.525 |
| Mean | 30 | 45% | 2.14 |
Table 2: Incidence of citrus gummosis in ten major citrus growing orchards of Ethiopia, 2012. atree decline on a 0-4 scale where 0= no symptom and 4=decline of the tree 75-100%, including dead tree [9].
Infections of tree parts
Gummosis was more frequent on scions (trunk and branch) 66.96% than rootstocks 33.02% on the surveyed orchards. And from the scions cankers/gummosis were frequently recorded from the trunk 44.81% than the branch 22·17% (Table 3). Alvarez et al. [7] reported that the citrus Cankers were more frequent on scions than rootstocks 92·4 and 7·6% of respectively. The disease mainly affected mature citrus trees; Diseased trees showed cankers and gum exudations mainly on above-union parts, especially on the major limbs, whereas rootstocks generally remained healthy.
| Location | Root stocka (%) | Trunkb (%) | Branchc (%) |
|---|---|---|---|
| Tony farm | 13 | 14 | - |
| Hurso | 14 | 27 | 19 |
| Erer | 21 | 25 | 16 |
| Gota | 1 | 7 | 1 |
| Fetule | 13 | 12 | 7 |
| Nura Era | - | 3 | 1 |
| Merti | 2 | 1 | 2 |
| Tibilla | - | - | - |
| Melkassa | 3 | 6 | - |
| Koka area | 3 | - | 1 |
| Total | 70 | 95 | 47 |
| Percentaged | 33.02 | 44.81 | 22.17 |
Table 3: Occurrence of Phytophthora -cankers on root stock, trunk and branch of citrus trees in ten major citrus growing orchards of Ethiopia, 2012. aRootstock from ground to union line; bTrunk refers to main stem/branch; c all branches and sub-branches from the trunk and percentages of 212 infected trees of 10 surveyed orchards.
The dominant status of P. citrophthora compared to other Phytophthora species on citrus is favored by environmental conditions [11,12]. The outbreak of this disease could be the variation in environmental factors and changes in citrus cultural practices. Inoculums raised from the soil and move to the trunk and branches possibly due to poor cultural practice conducted at the orchards during harvesting, irrigating and pruning. Likewise, the particular and most importantly this pathogen were spread during harvesting when climbing to the tree. Since harvesting is conducted without ladder when the harvester tries to climb to harvest he/she holds the inoculums with the soil on their feet’s and hands. During irrigation, weeding, pruning etc. actions fever the pathogen to reach on the branch of the tree. In addition; probably some insects and animals have had contribute for the spread of the pathogen to the upper branch. The disease usually attacks the plant when soil comes into contact with the scion, or when a tree is planted in a basin which may be flooded during irrigation, allowing the fungus to reach the scion [13-15].
Correlation between tree decline and altitude
The analysis result showed that branch incidence was highly and positively correlated with trunk incidence (r=0.889), root stock (r=0.776). Root stock incidence was strongly and negatively correlated with altitude (r=-0.626). Altitude was also negatively correlated with tree decline (r=-0.319) with weak association. These relations clearly mean when altitude increases incidence on root stock, branch and trunk as well as tree decline also decreases. The citrus gummosis was observed in 79% of the 90 orchards visited with high prevalence being observed in low altitude areas [16]. And when trunk incidence increases branch incidence also increases which indicates that citrus gummosis can affect branches if appropriate control measures aren’t timely designed.
Disease severity index
The overall result of disease severity index showed that Erer and Hurso orchards were highly affected by the disease. The lower disease severities were recorded at Tibilla (22.917%) followed by Melkassa (29.17%) and Merti (38.33%) and the highest disease severity recorded at Erer (91.04%) followed by Hurso (71.89%), Fetule (65.625%) and Koka (63.125%) (Figure 2). Hebb and Sonoda [16] reported that in Florida a survey of 18 grape fruit groves showed that 3% to 74% of the trees were affected by citrus gummosis. Similarly Jagtap et al. [15] also reported that in Marathwada region of Maharashtra state, all 103 sweet orange orchards surveyed had shown an average disease incidence of 38.83%.
In the soil analysis result all citrus orchards show clay texture except Melkassa which is loam relatively with low PH (7.52). This clay nature of the soil favors the pathogen by keeping the soil moisture for the long period of time and breaking of spore dormancy to be infective. Leonard and Nathan [17] reports about the spore survival mechanism were that the fungus survives in the soil as a thick-walled spore capable of withstanding extremes in both moisture and temperature. Clay nature of the citrus orchard and optimum temperature for the development of the spores of the pathogen as well as weak management of the orchards made the disease highly sever in the area.
Incubation and isolation
It was consistently isolated from diseased barks on modified selective medium PAVD (Figure 3). The Phytophthora spp. grown on V-8 medium and PDA produced sporangia abundantly in soil extracts. Morphological aspects of isolates were used in accordance with the descriptions made by Drenth and Sendall [18] (Figure 3). Isolates from the bark tested for growth on the selective medium at 23oc of temperature shows a white cottony colony after 72 hrs. But after seven days of incubation different types of spores were become visible on the culture under microscope including chlamydospores, sporangia and hyphal swellings. Isolations from the bark of citrus tree revealed the presence of the fungus Phytophthora spp, the cause of citrus gummosis, affecting root crown and the branch. All thus features were also described by Farih, et al. [19], Ali and canihos et al. [20], Alvarez, et al. [7].
Citrus gummosis (Phytophthora spp.) is an extremely destructive disease that can cause drastic reduction in yield and even a total decline of the orchards, if left unchecked. In the surveyed orchards there was 70% linkage between the die back and gumming of the branch. this indicated the dieback will decrease with the decrease of gumming of the main branch and infection of the main branch will also decrease with the decrease of contamination of the inoculum from soil to the main branch. Citrus gummosis was observed in 90% of the orchards to a different extent. An average citrus gummosis incidence reaches up to 45% in the orchards. But it can be reversed with the integration of fungicides, dimethomorph (the data is not observed), by avoiding excessive overwatering, poor drainage and soil structure, proper bud union (avoiding a bud union at soil surface or less than 15 cm above soil line those which enhances the disease gummosis. In general if the inoculums of the disease from soil were kept away through by any means to the rootstock and the trunk, the disease pressure will be minimized.
I would like to acknowledge the Ethiopian Institute of Agricultural Research (EIAR) for its support during the research and Rural Capacity Building Program (RCBP) for the fund.