Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Research Article - (2023)Volume 8, Issue 3
Objective: Colorectal Cancer (CRC) is a foremost global health concern and remains one of the major causes of cancer-related morbidity and mortality. It is the third most common cancer in adults after lung cancer and breast cancer worldwide. The theory that cancer originates from a subpopulation of tumor cells, named Cancer Stem Cells (CSC), they have important role of CSC in the initiation and maintenance of the tumor, as well as invasion, metastasis and therapeutic resistance. Among CSC markers, CD44 and OPN are two of the most investigated colorectal CSC markers and their proteins are introduced as the subpopulation with a greater tumorigenicity. This study aiming assessing the immunohistochemical expression of CD44 and OPN in colorectal adenomas and CRCs. And their relation between immunohistochemical expression of CD44 and OPN with tumor differentiation (grading), lympho-vascular invasion, perineural invasion, desmoplasia and TNM stage.
Method: This is a retrospective descriptive study that included sixty paraffin embedded blocks from the pathology laboratory, Suez Canal University Hospital. Paraffin blocks included (14 cases of colorectal carcinoma and 18 cases of colorectal adenoma). Paraffin blocks reviewed for clinicopathological prognostic factors and stained by CD44 and OPN, monoclonal antibodies by immunohistochemical method.
Results: The CD44 protein was overexpressed in 80% of CRC, while was positive (44.4%) in adenoma this difference was statistically significant. Also, in this study the difference between the expression OPN in CRC and adenomas was statistically insignificant.
Conclusion: CD44 is highly expressed in large number of CRC (80 of tumors). It is also significantly more expressed in CRC than in adenomas, suggesting a role of CD44 in CRC tumorigenesis and progression of adenomas into carcinomas. Our study also associated CD44 overexpression with both late TNM stage and lympho-vascular invasion.
Cancer Stem Cell (CSC); Osteopontin ; CD44; Immunohistochemical ; Colorectal Carcinoma (CRC); Diaminobenzidine (DAB); Horseraddish peroxidase; Lymphovascular invasion; Secreted phosphoprotein
Colorectal Cancer (CRC) is the third commonest cancer world- wide. It is also considered the third most frequent cause of cancer- related death usually due to the presence of liver metastases [1]. In Egypt, it ranks seventh among both genders [2]. Colorectal cancers are a group of diseases caused by genetic predisposition, nutritional habits, lifestyle, and environmental factors. The greatest proof of this is that the incidence of colorectal cancer is different in each country. More than 90% of colorectal cancers are adenocarcinomas. The remaining percentage of colorectal cancers are malignant carcinoid, lymphoma, neuroendocrine carcinoma, squamous cell carcinomas and other very rare types [3]. Cancer Stem Cells (CSCs) are thought to be closely related to the occurrence and development of tumors [4]. Numerous studies have shown that CSCs play an important role in colorectal cancer [5,6]. CD44 is a cell surface adhesion receptor that is widely expressed in most cell types, including epithelial cells, leukocytes, tumor cells, and vascular endothelial cells. The CD44 gene is located on chromosome 11 of the human genome [7]. CD44 is a marker of stem cells in colorectal adenocarcinoma. CD44 is a transmembrane protein which is also part of cell adhesion molecules that plays a role in cell-to-cell communication and interactions with the extracellular matrix and has a role in tumor development, proliferation, and metastasis in which affect the prognosis and survival [8,9]. Osteopontin (OPN) is a secreted glycophosphoprotein, encoded by the SPP1 gene, located on chromosome 4q13 [10]. While it is believed that binding of OPN to integrins regulate tumor cell migration, endothelial cell survival, migration and lumen formation in neovascularization, binding of OPN to CD44 receptors controls host-immune cell recognition, adhesion, transformation of normal to cancerous cells and tumor growth [11]. OPN thus influences hallmarks of cancer, for example, maintenance of proliferative signalling, resistance to cell death, replicative immortality, evasion of growth suppressors, neoangiogenesis, invasive growth and metastasis, deregulation of cellular energetics, avoidance of tumor destruction by the immune system and promotion of inflammation [12].
Although CD44 and OPN proteins have been tested as adhesion markers in colorectal adenomas and carcinoma previously. Their prognostic value has not been elucidated clearly [13]. This study aimed at assessing the immunohistochemical expression of CD44 and OPN in colorectal adenomas and CRCs. Also, it assessed the relation between immunohistochemical expression of CD44 and OPN with different prognostic parameters in CRC such as: Tumor differentiation (grading), lympho-vascular invasion, perineural invasion, desmoplasia and TNM stage. This helps us to use CD44 and OPN in the immunohistochemical panel of colorectal carcinomas as a diagnostic factor.
The aim of this study aimed at assessing the immunohistochemical expression of CD44 and OPN in colorectal adenomas and CRCs. Also, it assessed the relation between immunohistochemical expression of CD44 and OPN with different prognostic parameters in CRC such as: Tumor differentiation (grading), lympho-vascular invasion, perineural invasion, desmoplasia and TNM stage.
This study was carried out in the Pathology lab, Faculty of Medicine, Suez Canal University Hospital. It included 60 specimens divided into 42 specimens of CRCs and 18 specimens of colorectal adenomas which was chosen randomly. The sample size was calculated based on the following equation:
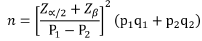
n = required sample size per group
Z(∝/2= 1.96 (The critical value that divides the central 95% of the Z distribution from the 5% in the tail).
Zβ = 0.84 (The critical value that separates the lower 20% of the Z distribution from the upper 80%.
Sections stained by hematoxylin and eosine stain to confirm the first diagnosis. All sections were assessed for the prognostic factors (Grading, lymphovascular invasion, perineural invasion, desmoplasia and TNM Stage).
Immunohistochemical technique
Immunohistochemical technique was manually performed according to the manufacture company in data sheet was manual, sections were cut at 5 microns and mounted on positively charged slides then dried in the oven at 56°C. Then the sections were deparaffinized by Xylene. The slides were processed through a serial alcohol for rehydration using (Absolute alcohol 90% and Alcohol 70%) and then rehydrated with distilled water. The slides were rinsed using distilled water for 5 minutes. The slides were placed in peroxidase block for 20 minutes and then rinsed with PBS three times 2 minutes every time. The application of primary antibodies: For CD44, prediluted, ready to use antibody, 100 μl of CD44 was applied to the slides for 30 minutes and for OPN which was titrated using a serial of dilutions to achieve optimal results, with a dilution of 1:50. Also,100 μl of OPN was applied to the slides for 30 minutes, incubated overnight in 4°C with the use of both positive and negative controls, then rinsed with PBS three times 2 minutes every time, then the slides incubated overnight in 4°C. We used the tonsillar tissue as a positive control for CD44 [14]. For OPN we used the normal gall bladder epithelium [15]. Then rinsed with PBS three times 2 minutes every time. 100 μl of HRP (Horseraddish Peroxidase) was used for 20 min then rinsed with PBS three times 2 minutes every time. Negative control slide was included in each run by omitting the primary antibody. Sections were completely covered with the working solution of liquid DAB, incubated for 5 minutes at room temperature for full color development. Slides were rinsed using distilled water and counterstained with Hematoxylin for 1 min. Then tissue sections were dehydrated by serial immersion in coplin jars containing series of graded ethanol, then xylol and mounted with DPX.
Evaluation of staining
CD44 expression: The staining of entire tumor-involved area was graded in terms of both extent and intensity [16]. The intensity of the staining was divided into four grades: (0: None, 1: Weak, 2: Moderate and 3: Strong). The extent of staining was divided into five categories: (0: ≤ 5%, 1: 6–25%, 2: 26–50%, 3: 51–75% and 4: 76-100%). Finally, we determined the score by multiplying the intensity and the extent of staining to produce a range of immunostaining scores from (0-12) as (0,1,2,3,4,6,8,9,12), the immunostaining was considered positive when the scores are ≥ 3 scoring of CD44 will divided into negative=(0-2), mild=(3-5), moderate (6-8) and strong (9-12) [14].
OPN expression: A scoring system related to the extent and intensity of immunostaining of tumor cells was used: The intensity of positive staining was scored as (0: Negative, 1: Weak, 2: Moderate and 3: Strong). The extent of positive staining was scored as: (1: (<10%), 2: (10–50%), 3: (>50-100%) and 4: (=100%). The final expression was determined by multiplying the intensity score by extent score, yielding a range 0,1,2,3,4,6,8,9,12. Scoring of OPN was divided into (negative (0-2), mild (3-5), moderate (6-8) strong (9-12) [17].
The sixty specimens have been examined for expression of CD44 and Osteopontin (OPN). Both markers have been correlated with clinicopathological features of adenoma (type and dysplasia) and CRC (grading, vascular invasion, perineural invasion, desmoplasia and TNM stage). The grade was determined according to WHO 5th edition grading and TNM stage was determined according to AJCC 8th edition.
The age of the sixty cases (adenomas and carcinomas) involved in this study was ranging from 26 to 80 years with mean (age) 57±10.7 (60 ±11.3 years for male and 49.5 ( ± 9.5) years for females in CRC) and 52 ± 17.18 (54 ± 18.8 years for male and 49 ± 15.3 for females in colorectal adenomas). The most common age group in carcinomas was those between 40 and 60 years, representing 51% of the study population. Whereas the most common age group in adenomas was that over 60 years representing 38.9% of study population. In this study the predominance sex was male in both adenoma and carcinoma, representing 66.7% in adenomas and 53.7% in CRCs. The distribution of histological subtypes among 18 colorectal adenoma specimens, 11(61.6%) were tubular subtype (Figure 1a), and only one case (5.5%) was of villous type (Figure 1b), while 6 (33.3%) were tubulovillous subtype (Figure 1c and Table 1).
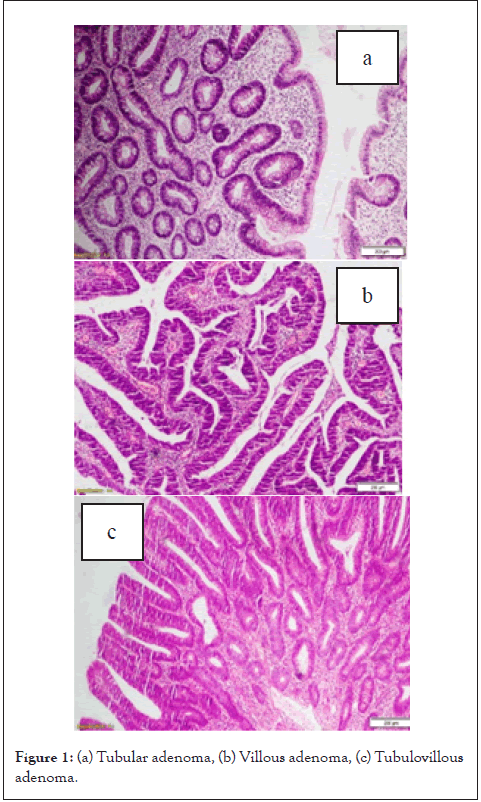
Figure 1: (a) Tubular adenoma, (b) Villous adenoma, (c) Tubulovillous adenoma.
| Features | Number of cases (42) | (%) |
|---|---|---|
| Grading | ||
| low | 39 | 93% |
| High | 3 | 7% |
| Vascular invasion | ||
| Positive LVI | 27 | 64.30% |
| Negative LVI | 15 | 35.70% |
| Perineural invasion | ||
| Free | 35 | 83.40% |
| Involved | 7 | 16.60% |
| Desmoplasia | ||
| Mild | 2 | 4.70% |
| Moderate | 25 | 59.50% |
| Sever | 15 | 35.80% |
| T stage | ||
| T1 | 8 | 19% |
| T2 | 21 | 50% |
| T3 | 8 | 19% |
| T4 | 5 | 12% |
| N stage | ||
| N0 | 28 | 66.60% |
| N1 | 9 | 21.40% |
| N2 | 5 | 11.90% |
| Distant metastasis | ||
| No | 36 | 85.70% |
| Yes | 6 | 14.30% |
| Tumor stage | ||
| Stage I | 1 | 2.30% |
| Stage II | 24 | 57.30% |
| Stage III | 12 | 28.50% |
| Stage IV | 5 | 11.90% |
Table 1: The clinicopathological characteristics of the studied colorectal carcinoma specimens.
Out of the 18 studied adenomas there were 13 (72.2%) showed low grade dysplasia, while 5 (27.8%) showed high grade dysplasia in this study. In our study most of tumors had cyto-membranous cytoplasmic expression of CD44 (Figure 2) and cytoplasmic expression, minor cases had membranous expression.
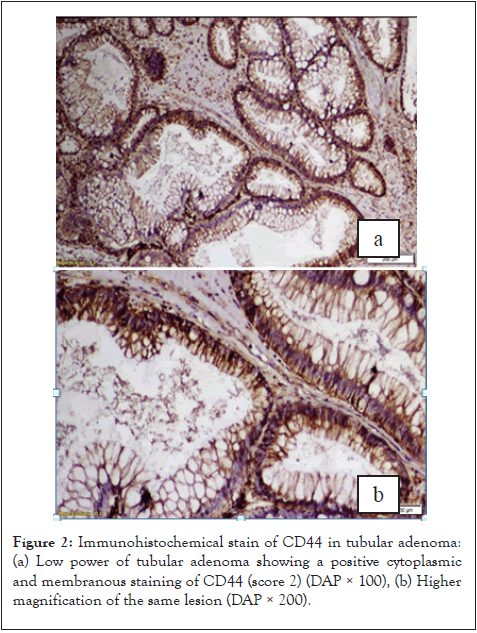
Figure 2: Immunohistochemical stain of CD44 in tubular adenoma: (a) Low power of tubular adenoma showing a positive cytoplasmic and membranous staining of CD44 (score 2) (DAP × 100), (b) Higher magnification of the same lesion (DAP × 200).
There were (44.4%) of studied colorectal adenomas had positive expression of CD44, whereas there were (81%) of studied CRCs cases had positive expression of CD44 with statistical significance (Table 2).
| Cases | P-value | |||
|---|---|---|---|---|
| Adenoma | Carcinoma | |||
| CD44. Expression freq. (%) | Negative | 10 (55.6%) | 8 (19.05%) | 0.012* |
| Positive | 8 (44.4%) | 34 (80.95%) | ||
Note: *Chi square test
Table 2: The frequency of CD44 expression in CRCs & adenomas specimens.
Forty percent of tubular adenomas were positive for CD44 in contrast to tubulovillous; 66.6% were positive. Whereas, the only one studied villous adenoma case was positive (100%); Unfortunately, this relation had no statistically significant. Regarding the grade of dysplasia of adenoma, there was no statistical difference between CD44 expression in different grades of dysplasia (p-value 0.065). According to our results the histologic grade divided into two groups low-grade (grade I-II) (n=39) representing 90.4% of all study population (Figure 3) and high-grade (grade III) (n=3) representing 9.6% of all study population.
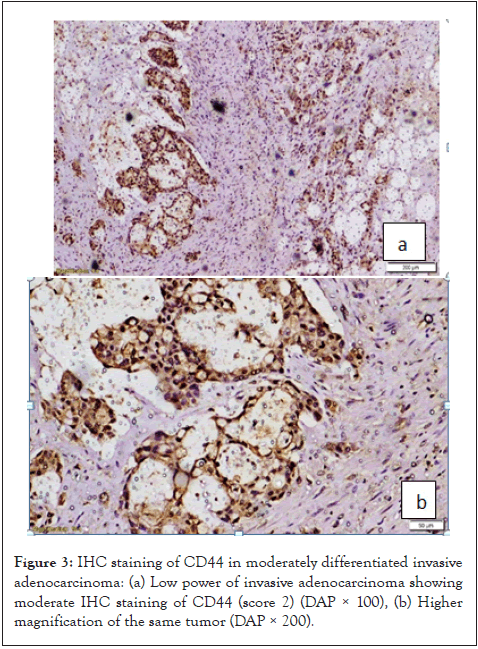
Figure 3: IHC staining of CD44 in moderately differentiated invasive adenocarcinoma: (a) Low power of invasive adenocarcinoma showing moderate IHC staining of CD44 (score 2) (DAP × 100), (b) Higher magnification of the same tumor (DAP × 200).
Unfortunately, the relation between CD44 expression and histologic grade was statistically insignificant (p=0.488). Although, most of low-grade tumors had positive CD44 expression (representing 80%) of all low-grade tumors while all high grade (100%) had positive CD44 expression. Regarding Lympho-Vascular Invasion (LVI): Positive CD44 expression was more expressed in tumors with positive LVI (95.8%) and their relation was statistically significant (p=0.049) (Table 3).
| Lympho-Vascular | CD44 expression | P-value | ||
|---|---|---|---|---|
| Invasion (LVI) | Number | Negative | Positive | |
| Negative | 15 | 7 (41.6%) | 11 (59.4%) | *0.049 |
| Positive | 27 | 1 (4.2%) | 23 (95.8%) | |
Note: *Fiher’s exact test
Table 3: The relation between CD44 expression and lymphovascular invasion by tumor cells of CRC cases (N=42).
Regarding, perineural invasion in this study there was no statistically significant relation between CD44 expression (p=0.669). The relation between immunohistochemical expression of CD44 and desmoplasia had no statistical significance (p=0.469). In the current study we grouped the four stages of the TNM staging system into two groups (stages I/II together and stages III/IV together) for more clear comparison. The relation between CD44 expression and clustered TNM stage (stages I/II and III/IV) was statistically significant (p<0.05) (Table 4), with significantly high CD44 expression in tumors with late stages (III/IV) compared with early stage (I/II).
| Clustered TNM stage | CD44 expression | P-value | ||
|---|---|---|---|---|
| Negative | Positive | |||
| I/II | 25 (59.5%) | 6 (24%) | 19 (76%) | *0.05 |
| III/IV | 17 (40.5%) | 2 (11.9%) | 15 (88.1%) | |
Note: *Fiher’s exact test
Table 4: The relation between CD 44 expression with TNM stage of CRC patients. (N=42)
In the present study revealed 18 cases of adenoma divided into (5 negative and 13 positive cases) according to OPN expression, and 42 CRCs cases divided into 11 negative and 31 positives for OPN. Most of studied cases have mainly cytoplasmic expression with minimal cases had cyto-membranous staining. We noticed that the large number of cases in both CRCs and adenomas had positive OPN expression (74% and 72% respectively) (Figure 4).
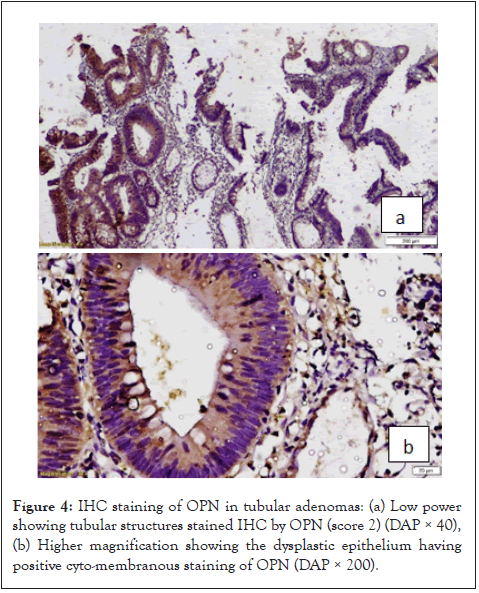
Figure 4: IHC staining of OPN in tubular adenomas: (a) Low power showing tubular structures stained IHC by OPN (score 2) (DAP × 40), (b) Higher magnification showing the dysplastic epithelium having positive cyto-membranous staining of OPN (DAP × 200).
There was no statistical difference between the expression of OPN in CRCs and colorectal adenomas (p=value 0.588). Regarding the histologic type of adenoma, there were 6 out of 11 of tubular adenoma were OPN negative and 5 were OPN positive. While the only one villous adenoma in our study was positive for OPN. Also, all tubulovillous type (6/6) were positive (p=0.167). According to dysplasia of colorectal adenomas all high-grade dysplasia specimens had positive expression for OPN (100%). Their relation was statistically significant (p=0.023).
In this study, there was 39 low grades including 71% had positive OPN staining and there are 100% of high-grade tumors were OPN positive (Figure 5), unfortunately, the difference was statistically insignificant (p=0.619).
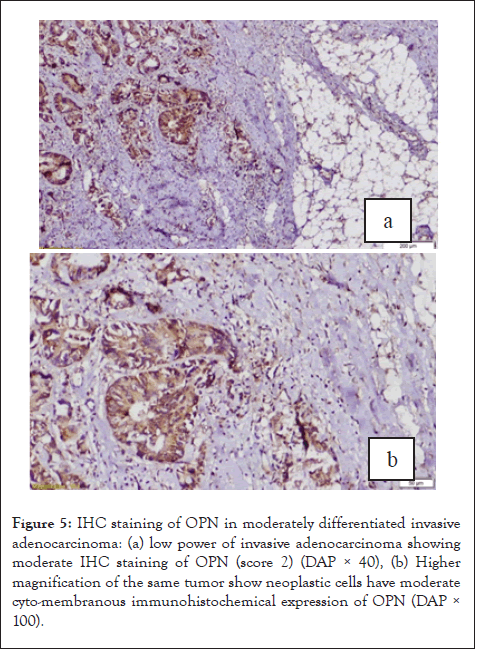
Figure 5: IHC staining of OPN in moderately differentiated invasive adenocarcinoma: (a) low power of invasive adenocarcinoma showing moderate IHC staining of OPN (score 2) (DAP × 40), (b) Higher magnification of the same tumor show neoplastic cells have moderate cyto-membranous immunohistochemical expression of OPN (DAP× 100).
Eighty percent of tumors with LVI were positive for OPN. While 66.7% of tumor with no LVI were positive for OPN. However, the relation between OPN expression and LVI was statistically insignificant (P=0.177). Regarding perineural invasion all specimens of positive perineural invasion (100%) had positive staining for OPN. While 68.7% of negative perineural invasion had positive OPN staining. The most common degree of desmoplasia in this study was the moderate one (25/42 cases) representing 60% of all study population. 19 cases (≈ 68%) of them having positive expression for OPN. While 2/42 cases showed mild degree of desmoplasia 100% of them were OPN positive. The relation between OPN and desmoplasia was statistically insignificance (p=0.723). Table 5 showed that there was no statistical significance between low stage tumors (I/II) and high stage tumors (III/IV) regarding OPN expression (p=0.591) (Tables 6 and 7).
| OPN expression | Cases | P-value | |
|---|---|---|---|
| Adenoma | Carcinoma | ||
| N=18 | N=42 | 0.588 | |
| Negative | 5 (27.8%) | 11 (26.8%) | |
| Positive | 13 (72.2%) | 31 (73.2%) | |
Table 5: The distribution of OPN expression in CRCs & adenomas.
| Lympho-vascular invasion (LVI) | Number | OPN expression | P-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Negative | 8 | 8 (33.3%) | 19 (66.7%) | |
| Positive | 3 | 3 (20.0%) | 12 (80.0%) | 0.177 |
Table 6: The relation between OPN and lympho-vascular invasion parameter of CRCs. (N=42)
| Clustered TNM stage | OPN expression | P-value | |
|---|---|---|---|
| Negative | Positive | ||
| I/II (N=25) | 6 | 19 | 0.591 |
| (59.50%) | 28.00% | 72.00% | |
| III/IV (N=17) | 5 | 12 | |
| (40.50%) | 29.4 | 70.6 | |
Table 7: The relation between OPN expression and TNM stage of CRC patients. (N=42)
Colorectal Cancer (CRC) is a foremost global health concern and remains one of the major causes of cancer-related morbidity and mortality in both developed and developing countries [18]. It is the third most common cancer in adults after lung cancer and breast cancer worldwide. Its incidence peaks at age 55-60 years [19]. In Egypt, CRC ranked the sixth, representing about 4% of total cancers in both sexes [20]. The incidence rate of colorectal cancer is 5.1% in males and 4.7% in females [21]. A variety of CSC markers have been proposed for CRC. Among them, CD44 and OPN are two of the most investigated colorectal CSC markers and CD44+ /OPN+ cells are introduced as the subpopulation with a greater colongenic ability and tumor initiation potential [22]. Our study conducted in 60 cases of CRC and colorectal adenomas to correlate CD44 and OPN immunohistochemical expression with clinicopathological finding such as grade of tumor, lymphovascular invasion, perineural invasion, desmoplasia and TNM stage. Many earlier works studied the association between clinicopathological characteristics of the CRC, with CD44 expression alone or in combination with OPN expression with controversy results. So, here we studied the relation between of CD44 expression and OPN expression, as the two are potential CSC markers with clinicopathological characteristics in CRC.
In the current study we observed that the CD44 was more expressed in CRC (80.95%) than in adenoma (44.4%) and the difference was statistically significant (p=0.012). This result was similar to who found a significant difference between adenoma and CRCs regarding CD44 expression [8,23]. This might be attributed to different CD44 expression in different stages in pathogenesis in colorectal carcinoma (colonic adenoma-carcinoma sequence) [24]. In our study 80.95% of CRC had positive CD44 expression. This was in contrast with study that showed that 57.1% of CRC cases had a positive CD44 expression. Also, 51% had a positive CD44 expression reported by study [25]. This different percentage might be attributed to different sample size in our and other studies (42 cases in this study and 250 cases in (8) study or may be due to the other studies included different types of colorectal carcinomas (eg: mucinous adenocarcinoma and signet ring adenocarcinoma) rather than adenocarcinoma type (NOS) used in this study [8,23].
Regarding lympho-vascular invasion in this study there was a significant relation between lympho-vascular invasion and expression of CD44 (p=0.048) [26]. Also demonstrated a statistically significant relationship between lympho-vascular invasion and CD44 expression. This may be attributed to tumors with high percentage of CSCs may have the capability to develop tumor cell migration and motility. So subsequently an overexpression of the CSC markers is more expected in advanced cancers with +ve LVI. However, these results were in contrast with who showed that no statistically significant relation between CD44 expression and the lympho-vascular invasion in CRC (p=0. 19) [22]. Their results agreed with study their p-value was insignificant (p>0.5) [27]. This may be due to the differences in number of cases [25]. According to our findings there was a significant relation between CD44 and tumor stage (p=0.05). There was increase of CD44 expression with late stages than in early stages. Our findings were similar to who also found that there was significant relation between CD44 and the tumor stage [26]. These results accentuate the results who also found a significant association between CD44 expression and stage III and IV [28,29]. However there was no relation between CD44 expression and stage of the tumor [30]. Also, study showed that there was no significant relationship between CD44 and stage [8]. The controversial results of CD44 are common, it is mainly due to different isoforms of the protein and different population upon whom the study was concluded.
Osteopontin (OPN), is a phosphorylated sialic acid–rich non- collagenous bone matrix protein, belonging to Small Integrin- Binding Ligand N-linked Glycoprotein (SIBLING) family [31]. Our results showed no statistically significant difference between the expression of OPN in CRCs or colorectal adenomas (p=0.588) like that of study of Likui, et al.[32]. Suggesting that the OPN is an early event in adenoma carcinoma pathway. This study showed that there was no statistically significant relation between OPN expression and TNM stage of CRCs (p=0.591). These results were similar to who demonstrated non-significant statistical difference on estimating the IHC expression of OPN in different CRC stages (p=0.111) [33]. The above-mentioned results were in contrast with study who showed that the expression level of OPN was significantly correlated with TNM stage (p=0.0012) [34]. Observed high levels of OPN expression could promote tumor progression and cell survival through Akt activation. Moreover, OPN has been proved to regulate cell motility, invasion, and metastasis formation of tumor cells [32,33].
We evaluate in this study the relation between expression of CD44 and Osteopontin (OPN) in colorectal adenoma and adenocarcinomas (NOS) and their relationship with clinic- pathological prognostic criteria of the disease by using IHC technique. The CD44 protein was overexpressed in 80% of CRC. On the other hand, CD44 expression was positive in about (44.4%) of adenoma. The difference was statistically significant. When we categorized the TNM stage into early and late stages, we found that there was statistical relation between positive CD44 expression and late stages (III and IV). Also, we found there was statistical relation between CD44 expression and tumors with positive lymphovascular invasion. On the other hand, there was no relation between CD44 and Grade, perineural invasion or desmoplasia. Regarding OPN, it was expressed in 74% of CRCs and 72% of adenomas. Also, in this study the difference between the expression OPN in CRC and adenomas was statistically insignificant. Moreover, there was no relation between OPN expression or score and the clinicopathological criteria of CRC (grade, lymphovascular invasion, perineural invasion, desmoplasia and TNM stage).
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Belel RAA, El Okda MO, Zidan AHM, Wagih HM (2023) Assessment of Immunohistochemical Expression of CD44 and Osteopontin in Colorectal Adenoma and Colorectal Carcinoma. J Can Sci Res. 8:543.
Received: 17-Jun-2023, Manuscript No. JCSR-23-25184; Editor assigned: 19-Jun-2023, Pre QC No. JCSR-23-25184 (PQ); Reviewed: 03-Jul-2023, QC No. JCSR-23-25184; Revised: 10-Jul-2023, Manuscript No. JCSR-23-25184 (R); Published: 17-Jul-2023 , DOI: 10.35248/2576-1447.23.8.543
Copyright: © 2023 Belel RAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.