Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2023)Volume 11, Issue 1
Tropospheric ozone (O3) acts as a phytotoxic secondary air pollutant and a potent greenhouse gas. O3 biomonitoring is a cost-effective approach compared to real time instrumental monitoring for identifying the potential effects of tropospheric O3 on crop growth and yield. In this study, eight Indian bean (Phaseolus vulgaris L.) genotypes were assessed for O3bio-monitoring potential along with the pre-identified O3-sensitive (S156) and O3 resistant (R123) bio-indicator Phaseolus genotypes provided by the international cooperative programme on effects of air pollution on natural vegetation and crops (ICP Vegetation), United Kingdom. One set of replicates was treated with the O3 protectant Ethylene-Di-Urea (EDU) while the other set was left under ambient environmental conditions without any treatment. The genotypes exposed to ambient O3 exhibited 0%-40% foliar ozone injury, though the timing of appearance of foliar O3 injury symptoms was variable among the genotypes. Plants treated with EDU exhibited lower foliar injury. Foliar O3 injury symptoms exhibited a significant positive correlation with cumulative O3 exposure (R2=0.73). The lipid peroxidation in bean plants under ambient O3 was observed to be 10%-62% greater than that of plants treated with EDU. The total protein and total carbohydrate content were also 4%-43% and 7%-40% lower, respectively, in leaves of plants exposed to ambient O3 as compared to plants treated with EDU. The results of the study indicate that the identified O3 sensitive genotypes of Phaseolus vulgaris viz. C1 (EC-755298) and C3 (IC-49810) can be used as O3 bio indicators for biomonitoring studies during the period between October and January in New Delhi, India.
Tropospheric ozone; Phaseolus vulgaris; Ethylene diurea; Air pollution tolerance Index; Foliar ozone injury; Ozone biomonitoring
The growing pace of urbanization and industrialization has resulted in a significant rise of air pollution. Air pollution acts as a major threat to both human health and vegetation. Ground level O3 or tropospheric O3 is one such air pollutant that poses a great impact on both human health and plant productivity. It is a secondary air pollutant and acts as a greenhouse gas. Being a secondary pollutant, its key precursors are mainly Nitrogen Oxides (NOx), hydrocarbons, and Volatile Organic Compounds (VOCs) which react in the presence of sunlight or lightning to release O3. It is considered as one of the Short Lived Climatic Pollutants (SLCPs) and has an average lifespan of 22 (± 2) days. Globally, the amount of O3 in the troposphere has been increased by about 3% between 1979 and 2014 (US EPA, 2016).
Tropospheric O3 is widely identified as one of the most damaging air pollutants to vegetation due to its phytotoxic nature and its high prevalence in the agricultural areas. O3 and its precursors can travel for long distances and thus can affect the remote rural areas hundreds and thousands of miles away from its source of origin where agricultural practices are favored. Due to wind turbulence, O3 is deposited on the plant surfaces where its uptake occurs mainly through stomata. Stomatal uptake accounts for up to 30%-90% of the ecosystem O3 uptake, which is a major component of the total terrestrial dry deposition of O3. Plant growth reduction and foliar injury are the most noticeable direct impacts of O3 pollution. Typically, intervene foliar injury is prevalent on the upper surface of the older leaves. It can be observed in different patterns of dots and flecks with its colour ranging from bronze, purplish to brown or yellow. Often in O3 sensitive species, large lesions are formed all over the whole leaf area leading to the early die-off of the plants.
O3 can also affect plant physiology indirectly by altering its biochemical functions. O3 enters the plant through the stomatal pores and degrades to form Reactive Oxygen Species (ROS) in the sub-stomatal cavity. ROS readily reacts with the cellular proteins, lipids, and nucleic acids and causes cell damage resulting in decreased yield, accelerated senescence and cell death. Due to the continued increase in ozone concentrations and its negative impact on crop yield, there is a need to find solutions to this problem. Possible ways to minimize the effect of O3 on plants include chemical protection, including fungicides, insecticides, and antioxidants to mitigate the effects of O3. Among these, ethylene diurea (EDU) has been suggested to be an effective protectant of plants against the effect of O3.
With the discovery of N-[2-(2-oxo-1-imidazolidinyl) ethyl]-N′- phenylurea (EDU) by Carnahan, et al. the antiozonant property of EDU was widely studied and reported. EDU is known to reduce the extent of foliar injury and helps to delay premature leaf senescence, thus prolonging the vegetative growth and increasing the carbon allocation in the given plants. EDU is known to remain in leaf apoplast for almost 10 days suggesting a direct role in protecting plants from ozone with a need for repeated applications through foliar spray or soil drench to maintain its antiozonant effect.
The tropospheric O3 concentration over the Indian sub-tropical region has been increasing since pre-industrial times, and particularly over the past few decades, with the highest increase being in Delhi. Tropospheric O3 is one of the key air pollutants which impact the agricultural health and crop yield of the country. Many studies have already reported yield loss of important crops such as wheat, rice, maize, and soybean due to an increase in tropospheric O3 concentration over the decades.
One of the most cost effective approaches in checking the severity of certain types of pollution in a region is the use of plants as bio-indicators. The plants used for biomonitoring should be easily identified in the field and should be easy to handle for damage analysis. Also, such plants should have a wide range of distribution. Plants that produce highly specific symptoms in response to damage caused by certain types and concentrations of pollutants are preferable. Commonly used plants for air pollution biomonitoring includes mosses, lichens, ferns, algae, and crop plants such as Hordeum vulgare, Phaseolus vulgaris, Raphanus sativus and Pisum sativum.
Bioindicator plants have been used in Europe and North America for O3 biomonitoring. Different plants exhibit differential response to O3 stress according to the plant species (even ecotypes), the weather condition of the region, nutrient availability, soil characteristics, etc. Foliar injury on the leaves is the first observable symptom of oxidative stress on a plant due to O3 exposure. Plants showing such symptoms act as an indicator of O3 pollution in the region over time (whether it is improving or declining) and its impact on the vegetation. One of the active ozone biomonitoring plants is the sensitive (S156) and resistant (R123) genotypes of Phaseolus vulgaris (Bush bean, French dwarf bean). The ozone-sensitive (S156) and ozoneresistant (R123) genotypes of Phaseolus vulgaris have been identified at the USDA-ARS Plant Science Unit field site and recommended as bioindicator for ozone biomonitoring. The Phaseolus S156/R123 system have been identified as a potential tool for active biomonitoring of ambient O3 and their differential ozone response have been extensively studied. Due to the need for an effective biomonitor suitable for the Indian climate, we assessed the suitability of eight Indian genotypes of Phaseolus and compared these to the S156 (O3 sensitive) and R123 (O3 resistant) genotypes used in the ICP Vegetation programme.
Common bean or bush bean (Phaseolus vulgaris) is frequently used in the study of air pollution, especially O3 pollution. Various studies on air pollution using Phaseolus were carried out in the past years. Common bean is widely distributed in different geographical regions and can acclimate to tropical, subtropical, and temperate regions, which makes it an effective tool for research purposes. It also has a short growing period of 4 months within which it can show quantifiable symptoms such as foliar injury and yield loss, which indicates the O3 pollution in the region. The foliar injury due to oxidative stress in Phaseolus vulgaris is unique with distinctive red or bronze lesions in between the veins.
The objectives of the present study are-1) to determine the impact of ambient O3 exposure and ozone protectant on the development of Indian bean genotypes. 2) To compare the Indian Bean genotypes O3 biomonitoring potential with O3 sensitive (S156) and O3 tolerant (R123) bio monitors of bean genotypes, recommended for use within the ICP-Asia network.
Experimental site
The experiment was conducted at the net house situated at ecological garden, school of environmental sciences, Jawaharlal Nehru university, New Delhi, India (28.5402°N, 77.1662°E). During the experimental period between 4th October 2018 and 10th January 2019, the meteorological parameters including maximum and minimum temperature, relative humidity, rainfall, and ambient levels of tropospheric O3were recorded. The meteorological data was provided by the ambient air quality monitoring station installed by Delhi Pollution Control Committee (DPCC) under CPCB, India (Central Pollution Control Board) at R.K. Puram, New Delhi. The prevailing ambient O3levels at the experimental site were monitored every day for 8 hours (9:00 am-5:00 pm) during the crop growth period using the ozone analyzer (Figure 1) [1].
Figure 1: Ozone uptake and its effect on cellular functions.
Note: ROS=Reactive Oxygen Species; JA=Jasmonic Acid; SA=Salicylic Acid; ET=Ethylene; PCD=Programmed Cell Death.
Experimental crop
In the present study, eight Indian genotypes of Phaseolus vulgaris (common bean/french bean/green bean) along with the two preidentified O3 sensitive (S156) and O3 tolerant (R123) genotypes recommended for use within the ICP-Asia network were selected. The seeds of Indian genotypes were procured from National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India. Seeds of all genotypes were sown on 4th October 2018 in field plots (1.5 m2) prepared at the experimental site. Six plants of all selected genotypes were maintained as replicates. The recommended dose of fertilizers as Urea, Muriate of Potash (MOP), and Single Super Phosphate (SSP) for Nitrogen (N), Potassium (K), and Phosphorus (P) for common bean (120:60:60 kg/ha) was applied in two doses. 60:60:30 kg/ha of NPK was applied as a basal dressing during preparation of field and the remaining quantity of fertilizers was applied at twenty five days after sowing (DAS). Manual weeding was done two times over the entire crop growth period and experimental field was irrigated at regular intervals to maintain the uniform soil moisture (Table 1 and Figure 2).
| Serial no. | Name of the genotype | Abbreviation |
|---|---|---|
| 1 | S156* | S156 |
| 2 | R123** | R123 |
| 3 | EC-755298 | C1 |
| 4 | EC-755628 | C2 |
| 5 | IC-49810 | C3 |
| 6 | IC-199205 | C4 |
| 7 | EC-755326 | C5 |
| 8 | EC-755343 | C6 |
| 9 | EC-755342 | C7 |
| 10 | IC-47858 | C8 |
| Note: *ICP-Asia network, pre-identified O3 sensitive genotype; **ICP-Asia network, pre-identified O3 resistant genotype. | ||
Table 1: List of genotypes used in the experiment.
Figure 2:Schematic representation of experimental setup layout.
Experimental treatments
All genotypes were exposed to two treatments viz. ambient ozone levels (O3) and anti-ozonate or O3protectant EDU (Ethylene Diurea (EDU, N-[2-(2-oxoimidazolidinyl) ethyl]-N’-phenylurea) during the experiment. At the 4 leaf stage i.e., 20 Days after Sowing (DAS), a set of 3 plants per genotype were treated with EDU until the plant maturity at the intervals of 10 days. For the first three applications, the selected plants were administered with 100 ml of freshly prepared EDU (300 ppm) per plant in the form of foliar spray. For the remaining applications, the dose was increased to 200 ml EDU (300 ppm) per plant.
Foliar ozone injury assessment
The percentage of leaves showing visible foliar injury on each plant was calculated weekly by counting the number of injured leaves and calculating its percentage in accordance with the total number of leaves on the plant.
Foliar Injury (%)=(leaves with visible O3injury (>5% O3 injury)/total number of leaves) × 100
Growth parameters
The total number of leaves per plant, leaf area and plant height (length of the main stem), were assessed for all plants. Leaf area was non-destructively assessed at the flowering stage (32 DAS) by using paper impressions of all leaves on the plant. The number of leaves per plant and plant height were recorded at the pod stage (65 DAS).
Biochemical parameters
For the biochemical analysis of plants, random sampling of full matured leaves per genotype was done through destructive harvesting. Leaf extract pH was determined by homogenizing 0.5 g of leaf sample in 10 ml distilled water. The filtrate was obtained, and pH was recorded with the help of pH meter calibrated with standard buffer solutions pH 4.0, pH 7.0 and pH 9.0.
Relative Water Content (RWC) of plants was estimated using the method as described by. Three leaves per genotype per treatment were taken and leaf area of 4 cm2 was excised from each leaf sample. The Fresh Weight (FW) of the excised leaf sample was recorded and then the leaf samples were left overnight in petri plates filled with distilled water to become saturated. Turgid Weight (TW) of the leaf samples were obtained next day after first blotting them dry with a paper towel. Samples were then oven dried at 65â?? until a constant weight was achieved to give the Dry Weight (DW). The RWC was calculated by using the formula.

For total chlorophyll content estimation, DMSO method by was adopted. For this, 100 mg of fresh leaf sample of each genotype under each treatment was taken in triplicates. The leaf samples were incubated overnight with 7 ml of Dimethyl Sulphoxide (DMSO). The absorbance of the solution was measured at wavelengths 480 nm, 645 nm, and 663 nm. The chlorophyll content was calculated using Arnon’s equations as:
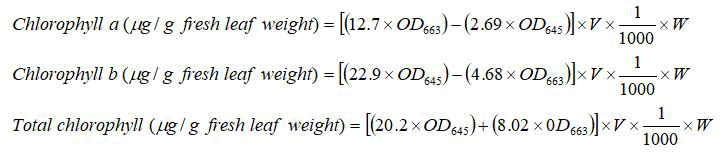
Where,
OD663=absorbance values at 663 nm;
OD645=absorbance values at 645 nm;
W=weight of leaf sample in mg;
V=volume of the solvent used in ml.
Ascorbic acid content of leaf samples was analysed according to the method given by. 0.5 g of fresh leaf samples were taken in triplicates for each genotype per treatment and homogenized in 10 ml 4% oxalic acid. The volume was made up to 35 ml with 4% oxalic acid. The mixture was centrifuged at 8000 rpm for 10 minutes and the supernatant was collected. 5 ml supernatant was mixed with 10 ml 4% oxalic acid and then titrated against the dye (V2). 5 ml working standard solution of ascorbic acid was mixed with 10 ml 4% oxalic acid and was titrated against the dye (V1). The ascorbic acid content was then calculated using the formula:
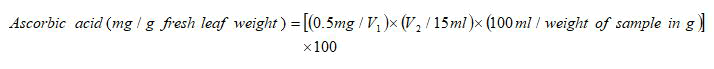
Where,
V1=Volume of the dye used when titrated against standard ascorbic acid solution (ml).
V2=Volume of dye used when titrated against sample (ml).
Lipid peroxidation assay was performed by following the method as described by Heath and Packer that 0.1 g leaf tissue was taken in triplicates and mixed with 0.5 ml 0.1% Tri Chloroacetic Acid (TCA). The mixture was centrifuged at 15000 rpm, for 10 minutes at 4°C. 1.5 mL of 0.5% Thiobarbituric Acid (prepared in 20% TCA) was added to 0.5 mL of supernatant, incubated for 25 minutes at 95â??, and then cooled in an ice bath. The absorbance was recorded for each sample at 532 nm and 600 nm and MDA content was calculated as:
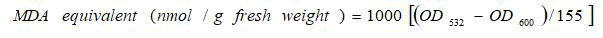
Where,
OD532=Absorbance at 532 nm wavelength.
OD600=Absorbance at 600 nm wavelength.
Air Pollution Tolerance Index (APTI)
APTI is an indicator of plant response to air pollutants formulated by Singh and Rao and is a function of key biochemical parameters. Air Pollution Tolerance Index (APTI) was calculated using the formula:
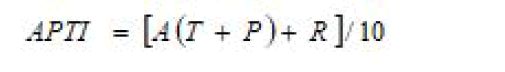
Where,
A=Ascorbic acid content (mg/g fresh weight),
T=Total chlorophyll content (mg/g fresh weight),
P=Leaf pH and
R=Relative water content (%).
Nutritional attribute analysis
Total protein content: Total protein content of leaf samples was determined by the method given by Lowry, et al. 300 mg fresh leaf samples were taken in triplicates and macerated in a prechilled mortar pestle with 10 ml 20% Tri Chloroacetic Acid (TCA). The homogenized mixture was centrifuged at 3000 rpm for 15 minutes. The pellet was collected and mixed with 5 ml 0.1% sodium hydroxide. The mixture was then centrifuged at 3000 rpm for 15 minutes. 0.5 ml supernatant was taken and mixed with 5 ml alkaline copper sulphate solution. The mixture was left in dark for 10 minutes. 0.5 ml folin-ciocalteau reagent was added and was again incubated in dark for 30 minutes. Absorbance for all samples was recorded at 660 nm. A reference curve of Bovine Serum Albumin (BSA) was used [2].
Total carbohydrate content: Total carbohydrate content was determined by anthrone method as described by Yemm and Willis. 200 mg dried leaf samples were taken in triplicates and ground to a fine powder. The ground leaf sample was boiled for 30 minutes in 20 ml distilled water. The mixture was filtered, and volume was made up to 50 ml with distilled water. 1 ml sample filtrate was mixed with 10 ml of 1 M anthrone reagent, boiled for 30 minutes in a water bath, and then cooled. The absorbance was taken at 625 nm and converted to total carbohydrate content using a standard curve based on glucose [3].
Yield attributes
Plants of each genotype per treatment were harvested at physiological maturity and the number of pods per plant was counted and their fresh weight was recorded.
Statistical analysis
Statistical analysis used two-way Analysis of Variance (ANOVA) to examine the effect of genotypes, treatment, and their interaction on each variable. All statistical tests were performed using IBM SPSS statistics software (SPSS Inc. version 28.0.0.0 (190) IBM Corp.). Pearson’s correlation test was used to explore the relationship between percent foliar injury and cumulative AOT40 at the time of appearance of ozone injury.
Meteorological conditions during the experimental period
The average temperature during the crop growth period at experimental site was maximum in the month of October 2018 (22.4°C) and minimum in January 2019 (10.4°C). The average relative humidity was maximum during January 2019 (65.5%) and minimum during October 2018 (57.6%). The average ozone concentration in the month of October 2018 (44.6 ppb) was highest and the minimum was recorded in the month of January 2019 (13.85 ppb). The ozone concentration exceeded the plant threshold limit (40 ppb) for 41 days, with an AOT40 of 995.84 ppb.h during the crop growth period. Very little rainfall was recorded in New Delhi during the experimental period. There was no rainfall recorded in the month of October 2018 and only 2 mm rainfall for two days was recorded in November 2018. A slightly greater rainfall of 13 mm for two days was recorded in the month of December 2018 (Figure 3) [4].
Figure 3: Daily mean ambient ozone concentrations (9.00 am to 5.00 pm) during the crop growth period.
Morphological parameters
The foliar ozone injury in each genotype was higher in plants exposed to AO3 as compared to EDU treated plants. The foliar injury range observed under AO3 was 8%-40% and that under EDU treatment was 2%-16%. The foliar injury was observed in the form of distinct intervene bronze or black colored spots on the upper leaf surface accompanied by leaf chlorosis. The foliar injury was observed to be first developed on genotype C3 followed by C2, C5, C4, and S156. The genotype which showed foliar injury later after 33 DAS was R123. The percent foliar injury was observed to be highest in C3 under AO3 (40.1% ± 2.1%) and lowest in C7 under EDU treatment (2.08%). In all genotypes, EDU treated plants showed 52%-94% lesser foliar injury than the non-treated plants of the respective genotype. C3 genotype treated with EDU exhibited the maximum decrease in foliar injury (94%) w.r.t. AO3 exposed plants of the same. Foliar O3injury symptoms also exhibited a significant positive correlation with cumulative O3exposure (R2=0.73) [5].
The maximum and minimum plant height was recorded in genotypes C1 (69.3 cm ± 32 cm) and S156 (14.3 cm ± 1.7 cm) under AO3 treatment, respectively. The maximum leaf area was observed in genotype R123 (67 cm2 ± 6.1 cm2) and minimum in C6 (3 cm2 ± 0.5 cm2) under AO3. Also, the total number of leaves per plant was maximum in genotype C7 (80.3 ± 9.5) and minimum in C6 (2 ± 1) under AO3. Plant height, number of leaves per plant and leaf area showed no specific trend among the genotypes when treated with EDU (Table 2, Figures 4 and 5).
| Geno types | Plant height (cm) | Leaf area (cm2) | No. of leaves/ per plant | Foliar injury (%) | Injury appearance day (DAS) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AO3 | EDU | AO3 | EDU | AO3 | EDU | AO3 | EDU | AO3 | EDU | |
| R123 | 8 | - | 67 ± 6.1 | - | 10 | - | 0 | - | 33 | |
| C1 | 69.3 ± 3.2 | 64.6 ± 7.8 | 10.7 ± 1.2 | 8 ± 1.9 | 40 ± 6.3 | 63.3 ± 5 | 30.6 ± 4.8 | 9.1 ± 4.8 | 20 | 37 |
| C2 | 20 ± 3.6 | 64.6 ± 7.8 | 8.5 ± 0.2 | 14.3 ± 0.9 | 27 ± 4.3 | 63.5 ± 5 | 40 ± 2.1 | 9.1 ± 4.8 | 17 | 20 |
| C3 | 20 ± 3.6 | 34.3 ± 1.7 | 5.33 ± 0.1 | 3.83 ± 1.1 | 27 ± 4.3 | 30.6 ± 3.8 | 40.1 ± 2.1 | 2.29 ± 2.2 | 16 | 23 |
| C4 | 20.3 ± 3.9 | 22.3 ± 1.2 | 59.25 ± 1.2 | 11.67 ± 3.1 | 29.6 ± 2.6 | 23.6 ± 2.8 | 32.6 ± 7.7 | 6.17 ± 6.1 | 18 | 25 |
| C5 | 14.6 ± 1.7 | 13.3 ± 2.4 | 10.66 ± 1.0 | 5.3 ± 1.3 | 22.6 ± 6.1 | 20.6 ± 6.9 | 34.2 ± 10.8 | 16.2 ± 10.6 | 17 | 28 |
| C6 | 15 ± 1.1 | 33.6 ± 1.4 | 3 ± 0.5 | 12.83 ± 2.3 | 2 ± 1 | 45.6 ± 2.8 | 17.6 ± 5.5 | 5.8 ± 3.6 | 19 | 25 |
| C7 | 64 ± 12.1 | 27.6 ± 5.4 | 6.5 ± 1.2 | 9.16 ± 0.6 | 80.3 ± 9.5 | 47.6 ± 4.9 | 8.1 ± 2.7 | 2.08 ± 2.08 | 24 | 25 |
| C8 | 31 ± 7.8 | 24.3 ± 3.1 | 8 ± 0.7 | 10.16 ± 3.9 | 56.6 ± 4.4 | 36.3 ± 4.6 | 16.02 ± 2.1 | 5.8 ± 3.1 | 20 | 23 |
| S156 | 14.3 ± 1.7 | 12.6 ± 1.7 | 48 ± 4.1 | 48.67 ± 9.8 | 50.3 ± 4.6 | 31 ± 6.08 | 27.8 ± 5.4 | 12.3 ± 4.1 | 18 | 18 |
Table 2: Plant height, leaf area, number of leaves, % foliar injury and days of appearance of injury of each genotype under different treatments.
Figure 4: Phaseolus vulgaris genotypes showing different extent of foliar ozone injury under AO3 concentration.
Figure 5: a) Foliar injury percentage in Phaseolus leaves under AO3 and EDU treatment. b) Relationship between foliar ozone injury and AOT40 at which first occurrence of injury occurs under AO3.
Biochemical parameters
The range of leaf extract pH was between 7.12 and 8.31. Under AO3 the minimum pH was recorded in genotype R123 (7.12 ± 0.04) whereas maximum pH was recorded in the leaves of genotype C7 (8.31 ± 0.11). A maximum increase in leaf pH was observed in C8 (4.33%) whereas a decrease of 4.47% was observed in S156 when genotypes were treated with EDU. The genotypes possessing highest and lowest RWC under AO3 were R123 (82.9% ± 1.52%) and C7 (68.1% ± 0.27%). However, there was not much variation in RWC among the genotypes and EDU application didn’t show any specific trend in increase or decrease of RWC. The maximum and minimum total chlorophyll content under AO3 was in C6 (2.88 mg/g ± 0.43 mg/g fresh wt.) and S156 (0.3 mg/g ± 0.29 mg/g fresh wt.), respectively. The highest amount of ascorbic acid under AO3 was found in genotype C2 (216 mg/g ± 5.5 μg/g fresh wt.) and lowest was found in S156 (79.9 μg/g ± 5.1 μg/g fresh wt.). The ascorbic acid content in genotypes under AO3 was higher (7.4%-59.4%) when compared to genotypes under EDU application except C4 and S156 (Table 3) [6].
| Genotypes | Leaf pH | RWC (%) | Total chlorophyll (µg/g) | Ascorbic acid (mg/g) | MDA content (nmol/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AO3 | EDU | AO3 | EDU | AO3 | EDU | AO3 | EDU | AO3 | EDU | |
| R123 | 7.12 ± 0.04 | - | 82.94 ± 1.52 | - | 0.87 ± 0.07 | - | 97.2 ± 7 | - | 2.05 ± 0.06 | - |
| C1 | 7.29 ± 0.02 | 7.59 ± 0.06 | 70.87 ± 1.09 | 69.97 ± 1.93 | 1.04 ± 0.06 | 1.44 ± 0.04 | 104.2 ± 3.2 | 97.0 ± 4.01 | 2.17 ± 0.08 | 1.940 ± 0.08 |
| C2 | 7.53 ± 0.06 | 7.54 ± 0.03 | 69.87 ± 1.62 | 74.93 ± 1.89 | 2.6 ± 0.08 | 2.63 ± 0.09 | 216 ± 5.5 | 160 ± 6.9 | 2.28 ± 0.05 | 1.98 ± 0.05 |
| C3 | 7.6 ± 0.01 | 7.5 ± 0.05 | 76.37 ± 1.68 | 70.24 ± 2.69 | 1.6 ± 0.05 | 1.24 ± 0.06 | 104 ± 5.0 | 86.8 ± 3.3 | 1.89 ± 0.05 | 1.63 ± 0.1 |
| C4 | 7.6 ± 0.04 | 7.46 ± 0.07 | 69.97 ± 1.73 | 70.4 ± 1.4 | 1.32 ± 0.08 | 1.05 ± 0.06 | 126.2 ± 0.8 | 142.4 ± 9.9 | 2.06 ± 0.05 | 1.75 ± 0.05 |
| C5 | 8.04 ± 0.09 | 7.91 ± 0.02 | 78.73 ± 1.97 | 78.26 ± 1.77 | 0.77 ± 0.08 | 0.85 ± 0.06 | 166.6 ± 7.0 | 128.0 ± 5.0 | 2.83 ± 0.07 | 2.04 ± 0.08 |
| C6 | 7.25 ± 0.04 | 7.4 ± 0.03 | 82 ± 1.65 | 80 ± 3.3 | 2.88 ± 0.08 | 2.0 ± 0.04 | 155.6 ± 7.9 | 79.6 ± 10.2 | 3.07 ± 0.04 | 2.8 ± 0.08 |
| C7 | 8.06 ± 0.09 | 7.9 ± 0.04 | 68.06 ± 0.27 | 65.4 ± 1.64 | 1.6 ± 0.2 | 1.06 ± 0.16 | 140.7 ± 3.7 | 88.25 ± 6.4 | 3.89 ± 0.05 | 2.4 ± 0.05 |
| C8 | 7.95 ± 0.04 | 8.31 ± 0.11 | 72.87 ± 2.8 | 70.53 ± 3.02 | 2.26 ± 0.1 | 0.66 ± 0.02 | 138.9 ± 1.9 | 102 ± 2.02 | 4.01 ± 0.06 | 3.07 ± 0.05 |
| S156 | 7.7 ± 0.03 | 7.37 ± 0.3 | 78.67 ± 1.5 | 82.13 ± 1.5 | 0.3 ± 0.01 | 0.64 ± 0.07 | 79.04 ± 5.1 | 79.9 ± 9.3 | 3.1 ± 0.4 | 2.5 ± 0.06 |
(MDA) content of plants exposed to Ambient Ozone (O3) and plants treated with EDU (EDU).
Table 3: Leaf pH, Relative Water Content (RWC), total chlorophyll content, ascorbic acid content and malondialdehyde (MDA) content of plants exposed to Ambient Ozone (AO3) and plants treated with EDU (EDU).
Lipid peroxidation
The Malondialdehyde (MDA) content was recorded higher in bean genotypes exposed to AO3 concentrations. Upon EDU application, MDA content was reduced by %-62% indicating a protective role of EDU against ozone stress. The maximum and minimum MDA content under AO3 was observed in genotypes C8 (4.01 nmol/g ± 0.06 nmol/g) and C3 (1.89 nmol/g ± 0.1 nmol/g), respectively. The highest decrease in MDA content upon EDU treatment was observed in C7 (62.08%) whereas lowest decrease was found in C6 (9.64%).
Nutritional attributes
A reduction in total protein content of the leaves was observed in all genotypes except C3 when exposed to AO3 concentrations as compared to plants treated with EDU. The highest and lowest decrease was exhibited by genotypes C4 (43.3%) and C6 (3.9%), respectively. The total carbohydrate content also exhibited reduction under AO3 compared to plants treated with EDU in all genotypes except C4. The highest decrease was observed in genotype C1 (40.4%). On contrary to this, genotype C4 exhibited an increase in total carbohydrate content by 56.8% [7]. (Figure 6).
Figure 6: Total protein and total carbohydrate content in leaves of Phaseolus vulgaris under AO3 and EDU treatment.
Yield attributes
Both Above Ground Biomass (AGB) and Below Ground Biomass (BGB) were higher in most genotypes under EDU treatment when compared with non-treated plants. The highest decrease in total biomass under AO3 compared to EDU treated plants was shown by C3 (57.77%) whereas C5 and S156 exhibited an increase in total biomass. There was a decrease in the total number of pods per plant (23.33%-79.19%) in the AO3treatment compared to EDU in all genotypes (except for C3). Similarly, the total weight of pods per plant was reduced in most genotypes in the AO3 treatment compared to EDU (again with the exception of C3). The highest number of pods and maximum average weight of pods per plant was observed in R123, with 12 pods per plant weighing an average of 7.23 g under AO3 treatment (Table 4).
| Genotypes | Total protein content (mg/g) | Total carbohydrate content (mg/g) | No. of pods/plant | Weight of pods/plant (g) | ||||
|---|---|---|---|---|---|---|---|---|
| AO3 | EDU | AO3 | EDU | AO3 | EDU | AO3 | EDU | |
| R123 | 0.78 ± 0.05 | - | 0.30 ± 0.02 | - | 12 | - | 7.23 | - |
| C1 | 0.53 ± 0.08 | 0.8 ± 0.03 | 0.34 ± 0.02 | 0.57 ± 0.02 | 3.6 ± 2 | 17.3 ± 7.3 | 1.3 ± 1.0 | 4.0 ± 1.9 |
| C2 | 0.36 ± 0.01 | 0.6 ± 0.01 | 0.27 ± 0.003 | 0.32 ± 0.01 | 2.6 ± 1.7 | 5.6 ± .4 | 0.19 ± 0.15 | 0.68 ± 0.2 |
| C3 | 0.79 ± 0.008 | 0.57 ± 0.01 | 0.28 ± 0.03 | 0.30 ± 0.01 | 3 | 2.6 ± 1.7 | 0.59 | 0.25 ± 0.21 |
| C4 | 0.34 ± 0.009 | 0.6 ± 0.005 | 0.69 ± 0.09 | 0.44 ± 0.02 | 0 | 0 | 0 | 0 |
| C5 | 0.37 ± 0.02 | 0.57 ± 0.01 | 0.30 ± 0.001 | 0.35 ± 0.009 | 2.3 ± 1.4 | 3 | 0.12 ± 0.07 | 0.17 |
| C6 | 0.49 ± 0.008 | 0.51 ± 0.01 | 0.25 ± 0.03 | 0.29 ± 0.03 | 0 | 0 | 0 | 0 |
| C7 | 0.58 ± 0.03 | 0.81 ± 0.01 | 0.26 ± 0.03 | 0.39 ± 0.02 | 4.6 ± 2.9 | 7.0 ± 1.0 | 0.66 ± 0.06 | 1.0 ± 0.4 |
| C8 | 0.60 ± 0.02 | 0.92 ± 0.03 | 0.29 ± 0.06 | 0.37 ± 0.01 | 8.6 ± 4.9 | 9.0 ± 5.0 | 1.5 ± 0.5 | 3.6 ± 2.3 |
| S156 | 0.48 ± 0.02 | 0.70 ± 0.009 | 0.27 ± 0.01 | 0.36 ± 0.01 | 7.3 ± 1.7 | 14.0 ± 1.0 | 0.8 ± 0.1 | 1.87 ± 1.3 |
Table 4: Total protein content, total carbohydrate content and yield attributes of different phaseolus genotypes in accordance with the treatment.
Air Pollution Tolerance Index (APTI)
Based on the four major parameters viz. ascorbic acid content, total chlorophyll content, leaf extract pH and relative water content, the APTI was calculated. The lowest APTI was derived for O3 sensitive S156 (68.83) indicating the poorer capacity of the genotype to tolerate the ozone phyto toxicity whereas C1 (83.12) and C3 (86.82) were the Indian genotypes exhibiting lower APTI. On the other hand, the highest APTI was recorded for C2 (164.20) and C5 (141.91). Based on the APTI values, the genotypes were categorized as O3sensitive and O3resistant genotypes (Tables 5 and 6).
| Genotype | Air Pollution Tolerance Index (APTI) | Category |
|---|---|---|
| R123 | - | Resistant |
| C1 | 83.12 | Sensitive |
| C2 | 164.2 | Resistant |
| C3 | 86.82 | Sensitive |
| C4 | 102.93 | Sensitive |
| C5 | 141.91 | Resistant |
| C6 | 121.2 | Resistant |
| C7 | 120.3 | Resistant |
| C8 | 117.77 | Resistant |
| S156 | 68.83 | Sensitive |
Table 5: APTI of test genotypes under AO3.
| Parameters | Genotype | Treatment | Genotype treatment |
|---|---|---|---|
| Foliar ozone injury | NS | 0.005 | NS |
| Leaf pH | <0.001 | 0.045 | <0.001 |
| RWC | <0.001 | <0.001 | <0.001 |
| Total Chl | <0.001 | <0.001 | <0.001 |
| Ascorbic acid | <0.001 | <0.001 | <0.001 |
| MDA | <0.001 | <0.001 | <0.001 |
| Protein | <0.001 | <0.001 | <0.001 |
| Carbohydrate | <0.001 | <0.001 | <0.001 |
| No. of pods | <0.001 | <0.001 | <0.001 |
Table 6: Two-way ANOVA for study of different genotypes of Phaseolus vulgar is under two different treatments (significance was observed at α=0.05, NS=No significant).
The results clearly indicate the ambient ozone adverse and ozone protectant beneficial effect on Indian bean genotypes growth, biochemical, nutritional and yield attributes, respectively. At the experimental site, the cumulative ozone exposure index above 40 ppb (AOT40) reached 995.84 ppb.h over a 98-days accumulation period. The highest average O3 concentration was recorded during the vegetative stage of the plants in October 2018, but the highest daily O3 concentrations occurred during the winter months (December 2018) during the pod filling stage [8].
Interveinal foliar injury in the form of brown or purplish speckles on the upper leaf surface was observed on all plants during this study. These symptoms were consistent with those specific to O3 toxicity, and it has been shown that the degree of these injury symptoms on plants can act as a good indicator of O3 stress. Phaseolus is well known for its O3 sensitivity and is widely used for bio-monitoring studies carried out by the international Cooperative Programme on the effects of air pollution on natural vegetation and crops (ICP Vegetation) and Phaseolus genotypes S156 and R123 were recommended as O3 sensitive and O3 resistant genotypes, respectively. We found similar distinctive injury symptoms in Indian bean genotypes under AO3 concentrations, however injury reduced in EDU treated plants. The first genotype to show foliar injury symptoms was C3, which exhibited foliar injury within the 3rd week after germination. All genotypes developed visible O3 injury symptoms during the vegetative stage [9].
Ascorbic acid acts as one of the first lines of defense against oxidative species and O3 toxicity in a plant. This leads to the reduction in Reactive Oxygen Species (ROS) and results in improvement of plant tolerance to oxidative stress. It could be expected that plants exposed to higher O3 concentrations possess a higher amount of ascorbic acid, assuming that production can be up regulate to detoxify the additional ROS. This would explain the higher amount of ascorbic acid content in plants under AO3 as compared to plants treated with EDU. The exceptions were genotypes C4 and S156, which showed poor non enzymatic defense and depletion of ascorbic acid pool which may have contributed to the O3 sensitivity of these genotypes. Continuous ozone exposure can lead to saturation of the ROS scavenging capacity of plants, leading to ROS accumulation in cells and eventually resulting in lipid peroxidation symptomatic of membrane damage causing adverse effects on their functional integrity. This explains the increased malondialdehyde content (product of lipid peroxidation) in all tested genotypes in the AO3treatment. Due to the loss of membrane integrity, the cell permeability increases leading to an imbalance in influx and efflux which would encourage an increase in RWC content of the leaves exposed to O3. However, no major deviations in RWC content of the leaves between EDU treated plants and AO3 exposed plants were seen in any of the genotypes. On the other hand, leaf pH of a plant is a direct indicator of the plant’s pollution tolerance capacity as pH mediates the physiological responses of the plants towards stress (Dash). O3 is a highly oxidative pollutant that cans readily oxidase cellular compounds which may decrease the pH of the leaf. However, in our study only three genotypes (namely C1, C6, and C8) exhibited a decrease in leaf extract pH under AO3 exposure.
The nutritional attributes (total protein content and total carbohydrate content of the leaf) of the test genotypes exposed to AO3 showed a significant decrease when compared to the EDU treated plants. This might be due to a higher rate of protein denaturation by accumulated ROS with subsequently increased conversion of protein reserves to amino acids under stress conditions. A study on mung beans supported our results and reported a 30% decrease in protein content of the plants exposed to AO3 as compared to the EDU treated plants. Some studies have reported that plants exposed to O3 during and after a thesis experienced reduction in leaf carbohydrate concentrations during the grain filling period; however, O3 exposure of the same magnitude, applied before a thesis, had a lower impact on the leaf carbohydrate content. Thus, the developmental stage is an important factor modifying the response of crops to O3 exposure in terms of both yield and carbohydrate metabolism.
When compared to the EDU treated plants, the plants which were exposed to AO3 experienced a significant decline in total number and the total weight of pods per plant. Such decline in plant yield may be due to the reduced photosynthetic activity and lower assimilates supply to reproductive organ development. There may be a lower accumulation of biomass in plants due to lower carbon assimilation and alterations in its partitioning. It can also lead to reductions in yield and modifications in crop quality. In India, previous studies have reported loss in yield of cotton, rice, wheat, soybean and amaranth due to the ozone exposure. The main reason behind yield loss in crops under the exposure to moderate O3 levels is thought to be due to the damage in photosynthetic apparatus by ROS action which inhibited the function of PSII. Chloroplasts are one of the primary attack sites of pollutants and many studies have reported that total chlorophyll content of the plants declines under O3 stress and is a clear indicator of pollution. This was confirmed in our study, which showed O3induced degeneration of chlorophyll in all bean genotypes and that was partially ameliorated by a protective EDU treatment.
Air Pollution Tolerance Index (APTI) is an empirical value that represents the tolerance capacity of plants to air pollution. APTI is well acclaimed in wide range of studies to assess the potential of plants to counter air pollution and being based on combination of four crucial parameters, APTI show more reliable results than any other analysis based on single parameter. Plants having high APTI values have higher tolerance capacity and can be used as sink whereas, plants with low APTI values can be used as bio indicators. Our results categorized the genotypes C1, C3, and C4 along with S156 to be sensitive genotypes with lower APTI values and higher foliar O3injury. These genotypes could be used as bio indicator species for O3bio monitoring. The genotypes which showed lower foliar injury and lower APTI values were C6, C7 and C8. However interestingly, genotypes C2 and C5 had higher APTI values indicating higher tolerance to O3pollution but also exhibited higher O3injury. These genotypes have the potential to be used as bio indicator plants in conditions of very high O3 pollution. The correlation between the APTI values and percentage of visible foliar ozone injury indicates that APTI value alone cannot be used as a sole predictor of O3sensitivity and must be taken in consideration with percentage of visible foliar injury.
The present study has shown the phytotoxic effect of ambient tropospheric O3on bean genotypes in the Delhi NCR region of India. Based on foliar ozone injury and APTI of the various genotypes observe din the present study, the genotypes C1 and C3 can be used as bio monitors in the Indian region in a similar way to the existing S156 and R123 genotypes. However in literature very less information is available regarding the O3 sensitivity of the Indian genotypes of Phaseolus vulgaris which can be used for local bio monitoring of O3 pollution. Further in depth studies using more Indian variants of Phaseolus vulgaris could complement the findings of this study, in addition to the identification of candidate plants for ozone pollution bio monitoring representing native species.
Delhi is one of the cities with the highest AO3 concentration in India, which is a major concern for human health and for the health of its vegetation. Quantification of O3 concentrations in ambient air is expensive and time consuming and biomonitoring studies offer a practical alternative for O3 quantification. We considered growth, foliar ozone injury and APTI of the various genotypes and concluded that genotypes C1 and C3 can be used as biomonitors in the Indian region in a similar way to the existing S156 and R123 genotypes. We also used the O3 protectant EDU to show that ambient air in Delhi has detrimental impacts on plant biochemistry, growth and development, and contributes to the O3 induced visible foliar injury in O3 sensitive genotypes.
[Crossref] [Googlescholar][Indexed]
[Crossref][Googlescholar] [Indexed]
Citation: Khumukcham P, Mina U, Hayes F, Smiti K, Yadav P (2022) Assessment of Indian Bean (Phaseolus vulgaris L.) Genotypes for Response to Ozone Protectant and Tropospheric Ozone Biomonitoring Potential. J Pollut Eff Cont. 10:351.
Received: 04-Jul-2022, Manuscript No. JPE-22-18418; Editor assigned: 18-Jul-2022, Pre QC No. JPE-22-18418(PQ); Reviewed: 01-Aug-2022, QC No. JPE-22-18418; Revised: 22-Mar-2023, Manuscript No. JPE-22-18418(R); Published: 30-Mar-2023 , DOI: 10.35248/2375-4397.22.11.359
Copyright: © 2022 Khumukcham P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.