Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2014) Volume 2, Issue 2
Chlorination is used in the worldwide to produce drinking water in the most developing world. Frequently lack of proper sanitation and pollution control increases the organic content in water sources thereby increasing the potential of trihalomethanes (THMs) formation. This paper evaluated the lifetime cancer risk and the hazard index caused by THMs contained in drinking water from four areas of Alexandria Governorate, northern of Egypt. Oral exposure and the health risk were estimated using a probabilistic approach. This was done by monitoring the free residual chlorine and the THMs content in drinking water and by obtaining the actual population characteristics. Population characteristics considered, among other variables, the water intake rate, the body weight and the exposure time, and were expressed as empirical frequency distribution curves. Results showed that the 95th percentile of the carcinogenic risk estimated for bromodichloromethane (BDCM) and dibromochloromethane (DBCM) were above the acceptable level of one in a million (10-6) even though in 26% of the cases tap water did not meet the minimum free residual chlorine content required by the Egyptian drinking water standard (0.35 mg/l). Until proper sanitation is implemented and water is managed integrally (in quantity and quality), the Egyptian government need to consider alternate disinfection systems otherwise may be review integrally its water supply policy in these areas.
Keywords: Trihalomethanes; Drinking water; Risk assessment
A water service is currently supplied to 99% of the Egyptian population. It is estimated that 99% of the distributed water is chlorinated. Chlorination of water has certainly contributed to reducing the incidence of gastrointestinal diseases such as cholera, typhoid fever, hepatitis, etc. Disinfection with chlorine has been recognized as one of the major public health achievements. However, appropriate levels of free residual chlorine in supply systems are needed to protect health and chlorine disinfectant is added to sources that are becoming polluted. The addition of chlorine reduces microbial risk but poses chemical risks when disinfection by-products (DBPs) are formed. DBPs occur when chlorine reacts with natural organic matter (humic and fulvic acids) presents naturally in water [1].
Natural organic matter, commonly measured as total organic carbon (TOC) is the organic precursor, while bromide ion is the inorganic one.
DBPs found in chlorinated water, trihalomethanes (THMs), which include chloroform (CHCl3), bromodichloromethane (CHCl2Br), dibromochloromethane (CHClBr2) and bromoform (CHBr3), has been widely studied because they are consid ered potentially carcinogenic [2]. In addition, recent studies suggest that they also produce reproductive disorders [3] if ingested during pregnancy [4-6]. Therefore, water utility managers try to reduce their formation while maintaining a free residual chlorine content that should be enough to inactivate microorganisms and prevent their re-growth in the distribution system. To control health risks caused by THMs, several countries have established a maximum content in drinking water. In the USA, the US EPA has set a value of 0.08 mg/l, in the United Kingdom and Canada [7] the limit is 0.10 mg/l, and also in Egypt it is 0.10 mg/l [8]. However, in Egypt, it is evidence that; water sources are becoming polluted due to a lack of sanitation raises concerns about the possible presence of DBPs in drinking water, so the risks are bigger because most of the population consumes tap water instead of bottled water.
Chlorination, the most commonly used method to disinfect tap water, has led to a sharp decrease in both mortality and morbidity from many diseases known to be waterborne [9]. However, the presence of chlorinated disinfection by-products (DBP) in tap water is of concern from a public health aspect because they are suspected to be carcinogenic [10]. The most significant group of DBP formed during chlorination is the THM such as CHCl3, CHBrCl2, CHBr2Cl, and CHBr3. CHCl3is classified in Group 2B as a possibly carcinogenic to humans, based on limited evidence of carcinogenicity in humans but sufficient evidence of carcinogenicity in experimental animals [9]. CHBrCl2 is a weekly mutagenic and it has been classified as probably carcinogenic to humans, with sufficient evidence in animals and inadequate evidence in humans [9]. Between the four THMs found in drinking water, CHBrCl2 appears to be the most potent rodent carcinogen. CHBr2Cl and CHBr3 are classified in group 3 due to the inconclusive genotoxicity [9]. The second prevalent DBP group is haloacetic acids (HAAs). Aside from THMs and HAAs, many other compounds that comprising the DBP group have been found in treated waters, which include haloacetonitriles, haloketones, haloaldehydes, halopicrin, cyanogen chloride, halophenols and chloral hydrate and many others [9]. The main THM effects are cancer and adverse reproduction problems such as abortion, miscarriage, and retarded fetal development [11].
Chloroform concentrations measured in breath or blood after swimming and showering have been correlated with the activity time, and the concentration of this compound found in water and air [12]. Rafael et al [13] have reported that the THM concentration increases in blood as compared with their pre-activity blood levels in individuals after water-consuming.
The aims of this research to evaluate the life cancer risk and the hazard index caused by THMs in drinking water for four areas communities of Alexandria using field data to characterize the local population and the quality of the drinking water.
For this research, four communities located in the Alexandria governorates were considered: Abou Qir, Sidi Gaber (Siouf WTP and Mamoura WTP), Agimy and Amireya (K40 WTP and Noubaria WTP) with less than 2,000,000 total residents. Each community has its own source to supply water where chlorine is automatically added before water enters the distribution network.
The field study was divided into two parts. In the first one, an environmental study was performed to determine the quality of drinking water. In the second part, data to characterize the population of each community was gathered to use it for the human health risk assessment. Field data was gathered during a 4-week sampling program in May 2012.
Water samples were collected from each area of household water intake points and from tap water inside the houses, after passage through individual storage tanks. Sampling sites were selected and some criteria were taken in consideration: (a) the population size; (b) the direct supply from the municipal network and (c) the household’s agreement to participate in the monitoring of their water and in the survey to gather information for the risk assessment analysis.
The environmental study consisted of 24 sampling points in different households (tap water). The parameters measured were freeresidual chlorine (Cl2) and THMs.
The THM extraction and analysis was carried out according to EPA 551 method [14]. Free residual chlorine was analyzed using a friendly colorimetric kit (DPD4).
The monitoring of residual chlorine in water carried out in the morning and in the afternoon, at the household’s water intake point and tap water.
To characterize population exposure scenarios questionnaires were applied to 100 adults from the four areas to determine their: tap water ingestion habits, time of residence at the site and body weight, and were expressed as empiric distribution functions (EDFs) to describe the variability of those parameters. To evaluate the lifetime cancer risk (Rci) caused by the exposure to halogen compounds in drinking water, a risk assessment model was applied (equation 1) based on United States Environmental Protection Agency guidelines [15]. The non-cancer hazard quotient (HIi) was used (equation 2) to estimate the risk caused by chloroform as a secondary carcinogen.
 (1)
(1)
 (2)
(2)
Where Cai is the concentration of the trihalomethanes measured (mg/l) in drinking water for each community, IRa is the water ingestion rate (l/d), Sfi is the specific cancer slope factor (mg/kg.d)-1 for bromide compounds and RfDi is the reference dose for chloroform (mg/kg.d). The other exposure variables are EF, exposure frequency (d/y); ED, exposure duration (y); BW, body weight (kg) and AT, average exposure time (d).
Free residual chlorine
A total of 288 measurements were done at households’ in tap water after passing through the individual storing tanks. Only 73.6% of the measurements (Table 1) [16] in the tap water fulfilled the free residual chlorine content established by the Egyptian drinking water standard of 0.35 [8]. This is a serious concern about the safeness of water, particularly from a microbial point of view.
| Parameters | Value |
Weight of Population, 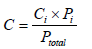 |
|
| Ci : Concentration of i region Pi : Population of water supply in the i region | |
| Ptotal : Total population of water supply in the i region | |
Exposure pathway of Ingestion,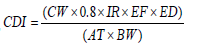 |
|
| Chronic daily intake, CDI (mg (Kg-day)-1) | |
| THMs concentration of drinking water, CW | |
| Intake quantity, IR | 2.5(liters day-1) |
| Average exposure time, AT | 70(year)×365(day/year) |
| Exposure during, ED | 70(year) |
| Exposure frequency, EF | 365(day year-1) |
| Body Weight, BW | Male : 64.8 ± 10(Kg) |
| Female : 56.3 ± 9.09(Kg) | |
| Absorptivity of body | 100% |
Table 1: References data and formula for exposure assessment [16].
According to Table 2, Amireya has the worse water quality supply and the major impact on water quality due to storage followed by Agimy, where all the values were less than or equal to the minimum enough level free residual chlorine of 0.35 mg/l. Less adverse conditions were observed in Abou Qir and Sidi Gaber.
| Chlorine Residual (mg/l) |
Abou Qir | SidiGaber | Agimy | Amireya |
| <0.1 | 3 | 0 | 9 | 14 |
| 0.1-0.3 | 6 | 3 | 23 | 18 |
| 0.35-0.6 | 12 | 9 | 22 | 16 |
| 0.65-1.0 | 30 | 16 | 10 | 10 |
| >1.0 | 21 | 44 | 8 | 14 |
| Average (mg/l) | 0.74 | 1.21 | 0.56 | 0.76 |
| Standard Deviation | 12.6 | 16.4 | 19.3 | 11.3 |
| Skewness | 0.57 | 0.87 | 0.82 | 0.64 |
Table 2: Free residual chlorine at household water in tap water sample.
THMs exposure levels
The total THMs concentration varied from 12 to 74 μg/l, always meeting the Egyptian drinking water standard of 100 μg/l. THMs concentration ranges for the different chemical species were as follows: CHCl3, 8 – 46.5 μg/l; CHCl2Br, 4 – 21.3 μg/l; CHClBr2, 0.0 – 6.9 μg/l and CHBr3 0.0-2.0 μg/l. Chloroform (CHCl3) was then the major specie, and was notably present in Agimy and Amireya as shown in Table 3. CHCl2Br was the most important brominates compound, CHBr3 being the least one. This is consistent with other studies [8]:
(a) Chloroform (Table 3 and Figure 1).
| Chloroform (µg/l) | Abou Qir | SidiGaber | Agimy | Amireya |
| <5 | 2 | 0 | 0 | 0 |
| 5-15 | 42 | 36 | 6 | 8 |
| 16-40 | 20 | 26 | 31 | 34 |
| >40 | 8 | 10 | 35 | 30 |
| Average | 26.4 | 23.3 | 29.8 | 32.4 |
| Standard deviation | 31.3 | 44.5 | 34.5 | 18.5 |
Table 3: Chloroform at household water in tap water samples.
(b) Dichlorobromomethane (DCBM) (Table 4 and Figure 2).
| Dichlorobromomethane(µg/l) | Abou Qir | SidiGaber | Agimy | Amireya |
| <5 | 48 | 44 | 0 | 0 |
| 5-15 | 16 | 18 | 44 | 52 |
| 16-40 | 8 | 10 | 28 | 20 |
| >40 | 0 | 0 | 0 | 0 |
| Average | 18.3 | 17.4 | 21.2 | 19.8 |
| Standard Deviation | 34.5 | 39.4 | 36.7 | 33.9 |
Table 4: Dichlorobromomethane at household water in tap water samples.
(c) Dibromochloromethane (DBCM) (Table 5 and Figure 3).
| Dibromochloromethane(µg/l) | Abou Qir | SidiGaber | Agimy | Amireya |
| <5 | 62 | 58 | 47 | 51 |
| 5-15 | 10 | 14 | 14 | 8 |
| 16-40 | 0 | 0 | 11 | 13 |
| >40 | 0 | 0 | 0 | 0 |
| Average | 11.3 | 12.3 | 14.8 | 15.2 |
| Standard Deviation | 44.2 | 50.3 | 55.6 | 59.6 |
Table 5: Dibromochloromethane at household water in tap water samples.
(d) Bromoform (Table 6 and Figure 4).
| Bromoform (µg/l) | Abou Qir | SidiGaber | Agimy | Amireya |
| <5 | 72 | 72 | 66 | 65 |
| 5-15 | 0 | 0 | 6 | 7 |
| 16-40 | 0 | 0 | 0 | 0 |
| >40 | 0 | 0 | 0 | 0 |
| Average | 1.3 | 1.2 | 3.9 | 4.2 |
| Standard Deviation | 14.3 | 16.7 | 22.3 | 24.7 |
Table 6: Bromoform at household water in tap water sample.
Risk estimation
The probabilistic health risk estimation [17] was estimated for drinking water ingestion. The cancer risk equation (1) was used for bromide compounds while the chloroform hazard quotient was estimated using equation (2). The empirical distribution functions developed for the ED, BW, Cai and IRa variables were used as inputs to the risk equations in order to obtain the risks as probabilistic functions. The hazard quotient for chloroform was estimated using the reference dose value given by IRIS [18], which is considered as protective to carcinogenic effects.
The 95th percentile of the probabilistic distribution functions (Table 7) calculated for the four areas communities showed in all cases that risk caused by chloroform and bromoform are acceptable (< than 1 for the first compound and < than 10-6 for the second one). However, the 95th percentile for bromodichloromethane and dibromochloromethane for tap water was greater than 10-6 for all communities. These results show that oral exposure to these compounds is higher than international acceptable levels, although content in water fulfill the Egyptian regulation.
| Bromoform (µg/l) | Abou Qir | SidiGaber | Agimy | Amireya |
| Chloroform | 6.82E-07 | 6.08E-07 | 6.07E-07 | 5.89E-07 |
| Bromodichloromethane | 1.27E-05 | 1.18E-05 | 1.22E-05 | 1.39E-05 |
| Chlorodibromomethane | 1.59E-05 | 1.51E-05 | 1.24E-05 | 1.33E-05 |
| Bromoform | 1.80E-05 | 1.80E-05 | 1.68E-05 | 1.65E-05 |
Table 7: The 95th percentile from the risk distribution functions.
This investigation included statistical analysis, epidemiology data and cancer risk analysis and assessment of THMs species in drinking water in Alexandria. It is more significant to establish an assessment procedure for the decision-making in policy of drinking water safety predominantly.
Specification the derive conclusions lie above from this study:
• The chloroform concentration is the major DBP species in the local regions of Cairo.
• The Southern region presented a high cancer risk (Amireya and Agimy).
• Residents of some districts were found to have a higher cancer risk through the oral ingestion of THMs. hazard indexes of THMs in different districts were found to be lower than unity, which did not indicate the noncancerous effects of THMs.
• Quantifying the risk factors is important for population and decision-making policy for drinking water safety. Fortunately, the Benchmark model and MCS and Risk supply the methodology were used for risk calculation. The standard for the total THMs species in Taiwan was 100 ug/l presently.
• We suggest that the standard be separated using separate dibromochloromethane, bromoform, chloroform, and bromodichloromethane standards. This may establish a control management for individual material to reduce the harmful risk. It displays the legislation limit values for different counties for DBPs levels.
• A methodology for decision-makers in formulating a procedure considering the economic, political, and feasible technology to reduce the standard value limits is necessary. An acceptable policy for safe drinking water and optimum social cost is the next objective of our study.
The techniques can be used for removal of THM compounds are activated carbon, enhancement coagulation, alternatives of disinfectants such as ozone, chloramine and chlorine dioxide.