Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2023)Volume 11, Issue 2
Background: The reduction of mortality in patients with sepsis depends on the early identification and treatment of at risk patients. No specific biomarkers have been found yet to reliably identify these patients.
Methods: We evaluated the association between mHLA-DR expression and the sepsis index (NCD64/MHLADR ratio) with the development of sepsis in 59 patients with stroke and 18 with traumatic brain injury. Both biomarkers were tested in whole blood samples at baseline and 3 days, 6 days, 9 days, 12 days and 15 days later.
Results: Most patients (71%) developed sepsis (4.2 ± 1.3 days after admission). On day 3, those subsequently developing sepsis had lower levels of mHLA-DR+(81.7% ± 16.2% vs. 88.5% ± 12.1%, p<0.05) and a higher sepsis index (0.19 ± 0.19 vs. 0.08 ± 0.08, p<0.01) tan those not developing sepsis. The mHLA-DR ratio slowly recovered before day 6, while the sepsis index remained raised in septic patients up to day 9 (p<0.05).
Conclusion: Periodic monitoring of the mHLA-DR expression together CRP and sepsis index may help to identify patients in the ICU at increased risk to develop sepsis.
Flow cytometry; Biomarker; Monocyte; HLA-DR; Sepsis
Sepsis, and particularly septic shock, causes high mortality rates in Intensive Care Unit (ICU) patients. Early identification of at risk patients might likely help to better tailor therapy in order to decrease the risk of death. Reversible immunodepression (i.e., immunoparalysis) has been associated with increased susceptibility to infections in critically ill patients. Monitoring the immune status of at risk patients might likely help to recognize immunoparalysis early and thus identify patients with an increased risk of infectious complications.
A number of molecules associated with immune failure have been studied [1]. The best biomarker to monitor immunoparalysis is Human Leukocyte Antigen-DR (HLA-DR) because it has the following characteristics: This molecule and expression is used within the immune system, specifically by monocytes, to present pathogen antigens and activation of T lymphocytes. Therefore, a lower expression on the surface of monocytes (mHLA-DR) is related to a lower capacity of the immune system to respond to an infection. Moreover, since it is a very early step in the immune response to pathogens, it is a very early biomarker of immunosuppression. HLA-DR has been proposed as a predictor of septic complications in critical conditions by many studies [2]. Another biomarker to monitor the immune status is CD64 molecule which is induced in neutrophils within a few hours after being in contact with bacteria. The increase of CD64 expression on neutrophils (NCD64) allows differentiation between resting and activated neutrophils and could be useful as a biomarker for infection monitoring.
Research on biomarkers that can enable more rapid detection of sepsis is essential to reduce mortality and morbidity rates. In the present study, we propose to investigate the role of HLA-DR expression on monocytes and of the sepsis index (ratio between nCD64 and mHLA-DR) as predictive biomarkers of sepsis [3].
Patients
Seventy seven critical neurological patients, admitted to the ICU of the Germans Trias i Pujol University hospital without infection, were included in a longitudinal prospective study over 24 months.
Severe neurological patients were chosen for the study because they are a relatively homogeneous group of patients who are admitted without being infected, whose stay in the ICU is prolonged and who usually become infected during their admission [4].
The inclusion criteria and exclusion criteria were as follows:
Inclusion criteria
• At least 18 years of age.
• Patients without infection but with serious neurological pathology.
Exclusion criteria
• Immunocompromised patients.
• Patients under 18 years of age.
• Patients who died within 24 h after admission.
Patients were monitored daily both clinically and analytically to detect sepsis and were classified as “septic” or “non septic”, as well as patients who develop septic shock according to the Sepsis-3 definition. In addition, the severity index was assessed by calculating the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the Sequential Organ Failure Assessment (SOFA) score on admission. Every patient was monitored for 28 days after admission.
This study was approved by the ethical committee of Germans Trias i Pujol hospital (PI-15-081) and all patients or their relatives gave their informed consent according to the Declaration of Helsinki [5].
Definitions
Infection was defined as a pathological process caused by the invasion of normally sterile tissues, fluids or body cavities, by pathogenic or potentially pathogenic microorganisms.
Sepsis (based on the Sepsis-3 conference) was defined as a "life threatening organ dysfunction caused by a dysregulated host response to infection". Furthermore, patients were classified according to the development of septic shock, defined as a “subset of sepsis in which underlying circulatory and cellular/ metabolic abnormalities are profound enough to substantially increase mortality".
Serious neurological pathology was defined as stroke and traumatic brain injury with decreased level of consciousness that required admission to an ICU. Immunocompromised patients were defined as patients diagnosed with any type of primary or acquired immunodeficiency (e.g., HIV positive, immunosuppression, patients exposed to chemotherapy, radiation, or steroids during an extended period of time or at high doses), or with a pathology advanced enough to suppress defenses against infection, e.g. leukemia or lymphoma [6].
Samples
Samples were obtained in the first 24 h after admission to the ICU and the analysis was performed within 4 h after blood extraction. Blood extraction for each patient was repeated every 72 h for 15 days, unless an early termination was established due to exitus [7].
Flow cytometry staining
Blood samples from the patients were collected with EDTA anticoagulant. 100 μL of whole blood was used, which was stained with 15 μL of CD64 PE, 5 μL of CD15 APC, 2.5 μL of HLA-DR BV421, 2.5 μL of CD14 APCH7 and 2.5 μL of CD3 BV515 (all from BD Biosciences, San Jose, CA, USA). After staining, cells were incubated for 20 min in darkness at room temperature (22°C-24ºC) followed by erythrocyte lysis, performed using BD FACS Lysing Solution (BD Biosciences) for 7 min. After centrifugation (1.300 rpm 5 min) and washes with FACSFlow (BD Biosciences), samples were acquired using a BD FACSCanto II (BD Biosciences). A minimum of 10,000 monocytes were recorded per sample [8].
Flow cytometry calibration
To standardize the analysis, we used Rainbow Calibration Particles (6 peaks; BD Biosciences) which contain a mixture of particles of similar size with different fluorescence intensities. The particles were used according to the manufacturer's protocol and were reconstituted with phosphate buffered saline (BD Biosciences). Equipment voltages and the Mean Fluorescence Intensity (MFI) were adjusted for each fluorochrome on a daily basis in order to standardize the protocol reducing the existing inter test variability [9].
Flow cytometry analysis
MFI of CD64 on Neutrophils (NCD64), MFI of HLA-DR on Monocyte (mHLA-DR) and on Lymphocytes (lHLA-DR) were measured (Figure 1). In addition, the HLA-DR expression rate on monocytes was measured. For the analysis, we used HLA-DR expression on lymphocytes as negative control and CD64 on monocytes as a positive control. The analysis of cell subpopulations was performed using the FACS Diva version 6.1.2 software (BD Biosciences) [9].
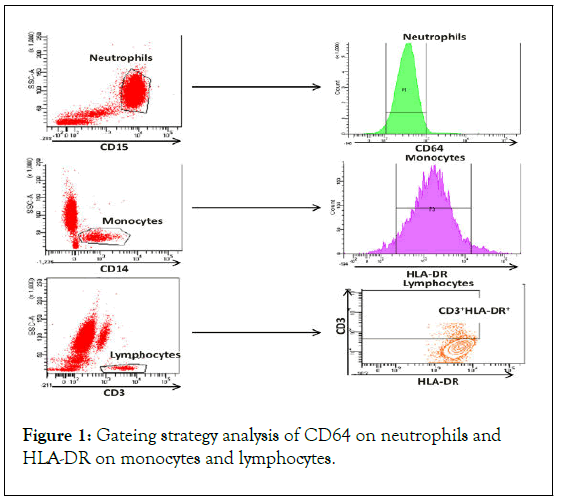
Figure 1: Gateing strategy analysis of CD64 on neutrophils and HLA-DR on monocytes and lymphocytes.
Neutrophils are characterized using the CD15 surface marker. CD64 expression is analyzed as Mean Fluorescence Intensity (MFI) on neutrophils. Monocytes are characterized using the CD14 surface marker. HLA-DR expression is analyzed as MFI and percentage over the monocyte subpopulation. Lymphocytes are characterized using the CD3 surface marker. HLA-DR expression is analyzed as MFI and percentage over lymphocytes.
Plasma analysis
C-Reactive Protein (CRP) was analyzed in plasma samples of patients at each time point using AU-5800 (Beckman Coulter, Brea, CA USA) immunoanalysers [10].
Statistical analysis
Quantitative variables are presented as the mean ± SD and qualitative variables as percentages and numbers. Normality criteria were determined using the shapiro wilk test. The Mann whitney U-test was used to compare differences in CRP, lymphocyte count, HLA-DR expression rate, MFI of mHLA-DR, HLA-DR Index, and sepsis index between outcome groups at each time point. In order to evaluate more stable biomarkers, we assessed the HLA-DR Index which was defined as the ratio between MFI of mHLA-DR and lHLA-DR. Moreover, we analyzed the sepsis index, defined as the ratio between nCD64 and mHLA-DR, as previously reported by other authors.
Analyses of HLA-DR index and sepsis index were performed after logarithmic transformation given that the distribution was evaluated as log normal [11]. Unadjusted and adjusted linear regression were used to evaluate mHLA-DR in relation to different time points before and after infection. Linear mixed models for repeated measurements were used to evaluate the dynamic variation in mHLA-DR and sepsis index at different time points, unadjusted and adjusted for gender and age.
Predictive values of the candidate biomarkers were investigated through Receiver Operating Characteristic (ROC) curves. Based on these curves, cut off values for relapse prediction were assessed for each potential biomarker. Statistical significance was set at p<0.05.
Figures show means ± SEM. The statistical package for social sciences (SPSS/Windows version 15.0; SPSS Inc, Chicago, IL, USA) and the software program Graphpad Prism (5.0 version; Graphpad, La Jolla; CA, USA) were used to perform statistical analyses [12].
Patients
Seventy-nine patients were selected for the study, of which two were not eligible for further analysis due to exitus before 72 h from admission. Thirty-six patients (71%) developed sepsis during admission and there was clinical suspicion at 4.2 ± 1.3 days after admission. Clinical and demographic characteristics of patients are shown in. The severity indicators showed a high APACHE II score of 21 (SD: 7) and a SOFA score of 7 (SD: 4). According to the sepsis stratification, patients who developed sepsis during the follow up had higher basal APACHE II and SOFA scores (APACHE II score: Septic patients: 22 ± 6, non septic patients 19 ± 9; p=0.05; SOFA score: Septic patients: 8 ± 3, non septic patients: 4 ± 3; p<0.001) [13]. Patients who developed sepsis spent more time in the ICU than those that did not develop sepsis during the follow up (septic patients 25 ± 15 days; non septic patients: 10 ± 7 days, p<0.001). In addition, mechanic ventilation was required for a longer duration in case of septic patients (septic patients: 17 ± 13 days, non-septic patients 4 ± 8 days, p<0.001). There were no differences due to gender, age, and comorbidities or in mortality between groups. Thirty-six patients (71%) from the septic group showed positive blood cultures and adequate antibiotic treatment was assessed in 41 patients (79%). Nine out of 55 septic patients developed septic shock during follow up (Table 1).
| Characteristics | Total cohort n=77 | Septic n=55 | Non septic n=22 | P-value |
|---|---|---|---|---|
| Female sex (no. of patients, (%)) | 26 (33) | 17 (19) | 9 (7) | 0.4 |
| Age (years), (IQR) | 54 ± 16 | 54 ± 16 | 56 ± 16 | 0.52 |
| Basal SOFA* score (IQR) | 7 ± 4 | 8 ± 3 | 4 ± 3 | <0.001 |
| APACHE ** II score (IQR) | 21 ± 7 | 22 ± 6 | 19 ± 9 | 0.05 |
| Median hospital days (IQR) | 21 ± 15 | 25 ± 15 | 10 ± 7 | <0.001 |
| Mechanic ventilation days (IQR) | 14 ± 13 | 17 ± 13 | 4 ± 8 | <0.001 |
| Comorbidities (no. of patients, (%)) | ||||
| COPD*** | 6 (8) | 4 (4) | 2 (2) | 0.79 |
| Smoker | 25 (32) | 16 (18) | 9 (7) | 0.32 |
| Alcoholims | 15 (19) | 9 (11) | 6 (4) | 0.27 |
| Cardiopathy | 8 (10) | 6 (6) | 2 (2) | 0.81 |
| Chronic kidney disease | 7 (9) | 5 (5) | 2 (2) | 1 |
| Cirrhosis | 2 (3) | 2 (1) | 0 (0) | 0.36 |
| Exitus (no. of patients, (%)) | 14 (18) | 11(10) | 3(4) | 0.51 |
| Blood culture (no. of patients, (%)) | 36 (71) | NA | ||
| Adequate antibiotic treatment (no. of patients, (%)) | 41 (79) | NA | ||
| Shock septic (no. of patients, (%)) | 9 (12) | 9 (16) | NA | |
Note: SOFA=Sepsis-related Organ Failure Assesment scale, **APACHE=Acute Physiology and Chronic Health Evaluation, ***COPD=Chronic Obstructive Pulmonary Disease
Table 1: Demographic and clinical characteristics of patients.
Pro inflammatory and anti-inflammatory imbalance three days after admission
CRP was at higher levels on day+3 after admission (161.83 ± 133.42 mg/mL) than at the basal time point (71.90 ± 76.71 mg/mL) in all patients (Figure 2). In contrast, lymphocyte count, mHLA-DR rate, MFI of mHLA-DR, HLA-DR index, and sepsis index did not show any differences during the follow up. As shown in, the dynamics of CRP, sepsis index, and MHLADR expression over time differed between groups [14]. Septic patients showed increased levels of CRP (septic patients 182.9 mg/mL ± 132.9 mg/mL; non septic patients: 93.46 ± 117.6 mg/mL, p=0.030) and sepsis index (septic patients: 0.19 ± 0.19; non septic patients 0.08 ± 0.08, p=0.010) at day+3 after admission. In contrast, mHLA-DR rate was found to be decreased (septic patients: 81.7% ± 16.22%; non septic patients: 88.53% ± 12.13%, p=0.040). The mHLA-DR rate slowly recovered before 6 days, while the sepsis index remained higher in septic patients up to day+9. There were no differences in lymphocyte count, MFI of mHLA-DR, or the HLA-DR index between groups during the follow up.
Figure 2: Changes in measures of immune dysfunction by subsequent sepsis status.
CRP levels of total patients included for difference over time (p<0.05); CRP levels for differences between groups (p<0.05); Sepsis index values for differences between septic and non septic patients (p<0.05); expression rate of MHLA-DR on monocytes for differences between groups (p<0.05).
A decrease in HLA-DR expression and an elevation of sepsis index enhance the probability of critical patients developing sepsis
The HLA-DR monocyte expression was evaluated in ICU admitted patients without infection and monitored for 15 days after admission. Considering the time of the sepsis diagnosis in the analysis, the mHLA-DR monocyte expression rate in septic patients significantly varied over time before the sepsis diagnosis (p=0.001). As shown in Figure 3, the mean values of HLA-DR expression on the monocyte surface, measured as MFI HLA-DR on monocytes, were found to be decreased in septic patients and recovered after infection [15]. The mixed model interaction test showed that septic patients showed lower significant differences over time in the mHLA-DR monocyte expression; and the differences remained significant after multivariate adjustments for gender, age, and mechanic ventilation (p<0.019). In addition, the percentage of HLA-DR+ monocytes tended to differ in septic patients over time (p=0.09). Patients who presented a high sepsis index showed a higher risk of developing sepsis (OR: 2.71, p<0.002). Moreover, statistical differences were found in the sepsis index means over time in septic patients vs. non-septic patients (p=0.005).
Figure 3: Differences in the mean fluorescence of mHLA-DR on monocytes from septic and non-septic patients before infection.
Dynamic values of mean fluorescence intensity of MHLADR on monocytes from septic (pink) and nonseptic (blue) patients during 15 days of follow up over time.
MHLA-DR expression rate presents higher specificity and less sensitivity than the sepsis index and CRP levels to classify septic patients
As seen in the ROC curve analysis, CRP levels were significant predictors of the development of sepsis, followed by the mHLADR expression rate and MFI of mHLA-DR, while lymphocyte count (p=0.286) and sepsis index (p=0.05) were not significant predictors. The Area Under the Curve values (AUCs) were the highest for CRP levels (AUC 0.765, p<0.001), followed by the mHLA-DR expression rate (AUC 0.666; p<0.001) and MFI of mHLA-DR (AUC 0.654; p<0.001) (Figure 4). Shows boxplots of CRP levels and mHLA-DR expression in septic and non-septic patients. To predict the development of sepsis, optimal cut offs were CRP levels>106.90 mg/mL (74%.19% sensitivity, 69.49 specificity), mHLA-DR expression rate<72%.80% (45%.31%sensitivity, 89%.47% specificity), and MFI of mHLA-DR<1882 (73%.53% sensitivity, 53%.76% specificity). The aim of this study was to identify biomarkers of immunoparalysis to predict which patients have an increased risk of developing sepsis at the ICU. The candidate biomarkers evaluated were [16].
• HLA-DR expression rate on monocytes.
• mean fluorescence intensity of HLA-DR on monocytes.
• HLA-DR index.
• Sepsis index.
Figure 4: ROC curves for potential predictive biomarkers of sepsis: CRP levels (a), the expression rate of HLA-DR on monocytes (b) and MFI of mHLA-DR (c) for the diagnosis of patients with higher risk to develop sepsis during their stay in ICU.
Our results showed that a pro inflammatory/anti inflammatory imbalance before infection produces an increased risk of developing sepsis in critical neurologic patients and this risk is increased in patients who remain hospitalized for long periods.
The initial injury which leads to their admission to the ICU may trigger an imbalance between the processes of inflammation and immunosuppression. These processes involve the activation of several intracellular pathways resulting in the production of pro inflammatory cytokines. In parallel, a Compensatory Antiinflammatory Response Syndrome (CARS) is activated as a temporary protective effect during the first hours after the injury. If this immunosuppressive state would be maintained over time, it could produce immunoparalysis, predisposing the patient to infectious complications and the development of sepsis [17].
The decrease in the mHLA-DR on circulating monocytes has been mostly accepted as a reliable marker of immunoparalysis in septic patients. This molecule reflects the loss of monocytes’ ability to present antigens and, consequently, to activate lymphocytes. Different authors have studied the association between expression levels of the mHLA-DR and prediction of sepsis. However, the results obtained in those studies were not conclusive. Differences in study design such as monitoring of time points, as well as the lack of standardization of flow cytometry protocols might be, in part, responsible for the discordant results [18]. To avoid this variability due to the technical procedure, we used a standardized flow cytometry methodology which can be easily transferred to other centers.
A prospective study in trauma patients without infection admitted to ICU showed that the mHLA-DR expression was decreased in patients who developed sepsis during the follow up. Similar results were found in our study involving patients with severe neurological injury where the mHLA-DR on day +3 of follow up was lower in patients that developed sepsis at later time points. To our knowledge, no other longitudinal and predictive studies have been performed analyzing mHLA-DR expression in ICU patients. These results collectively indicate that analysis of the mHLA-DR expression is a useful marker to monitor the immunocompetent status of the patients, and assess their susceptibility to the development of infections [19].
Regarding CRP levels, we observed higher values in patients who developed sepsis on day +3 of follow-up. At the same time point, the sepsis index value, which provides information regarding the pro-inflammatory and anti-inflammatory balance, was elevated, indicating that patients that developed sepsis suffered an imbalance due to an increase of inflammatory mediators and a decrease of HLA-DR molecules. While there was clinical suspicion at 4 days after admission.
Early diagnosis of the septic process is important to establish an adequate therapeutic strategy and increase survival rates [20]. Currently, the markers used in clinical practice to support the diagnosis of sepsis are CRP and Procalcitonin (PCT), but they have limitations. While CRP is highly sensitive, it lacks specificity for the diagnosis of sepsis; conversely, PCT is more specific but lacks sensitivity. Therefore, there is a need to look for biomarkers capable of providing a reliable diagnosis. In this context, the analysis of the evaluated biomarkers (mHLA-DR expression and sepsis index), showed changes before the diagnosis of sepsis. In contrast, no differences were found in those patients that did not develop sepsis during follow-up. The ROC analysis showed that the mHLA-DR expression rate was the biomarker with the highest specificity, and CRP the one with the highest sensitivity to predict sepsis.
Taking all the results mentioned above into consideration, we propose a diagnostic algorithm that could be implemented in the monitoring of critical neurological patients admitted to the ICU. First, immune-monitoring the sepsis index, CRP and mHLA-DR expression rate biomarkers at the admission and on day +3, to allow an early predictive stratification of susceptible sepsis patients. In this context, our preliminary results showed that the optimal cut-off values could be >106.90 mg/mL for CRP and <72.80% for mHLA-DR expression rate. Patients who would fulfill both criteria should be classified as potentially liable to develop sepsis. These biomarkers should be used in addition to other laboratory tests, such as PCT, and evaluated in the patient’s context to avoid misdiagnosis.
The present study has a number of limitations. First, it is a single-center study and the findings need to be confirmed in a larger and independent cohort. Moreover, we have only analyzed a specific group of patients (patients with severe neurological injury). The applicability of these biomarkers should be tested in different pathological contexts such as severe acute pancreatitis, trauma, burn or surgery.
We found a combination of biomarkers in peripheral blood able to stratify ICU patients with high risk to develop sepsis. These results have potentially important implications in the hospital care area, as the combination of parameters studied can facilitate the management of critically ill patients.
Regarding CRP levels, we observed higher values in patients who developed sepsis on day+3 of follow up. At the same time point, the sepsis index value, which provides information regarding the pro inflammatory and anti inflammatory balance, was elevated, indicating that patients that developed sepsis suffered an imbalance due to an increase of inflammatory mediators and a decrease of HLA-DR molecules. While there was clinical suspicion at 4 days after admission.
Early diagnosis of the septic process is important to establish an adequate therapeutic strategy and increase survival rates. Currently, the markers used in clinical practice to support the diagnosis of sepsis are CRP and Procalcitonin (PCT), but they have limitations [20]. While CRP is highly sensitive, it lacks specificity for the diagnosis of sepsis; conversely, PCT is more specific but lacks sensitivity. Therefore, there is a need to look for biomarkers capable of providing a reliable diagnosis. In this context, the analysis of the evaluated biomarkers (mHLA-DR expression and sepsis index), showed changes before the diagnosis of sepsis. In contrast, no differences were found in those patients that did not develop sepsis during follow up. The ROC analysis showed that the mHLA-DR expression rate was the biomarker with the highest specificity, and CRP the one with the highest sensitivity to predict sepsis.
Taking all the results mentioned above into consideration, we propose a diagnostic algorithm that could be implemented in the monitoring of critical neurological patients admitted to the ICU. First, immune monitoring the sepsis index, CRP and mHLA-DR expression rate biomarkers at the admission and on day +3, to allow an early predictive stratification of susceptible sepsis patients. In this context, our preliminary results showed that the optimal cut off values could be>106.90 mg/mL for CRP and <72.80% for mHLA-DR expression rate. Patients who would fulfill both criteria should be classified as potentially liable to develop sepsis. These biomarkers should be used in addition to other laboratory tests, such as PCT, and evaluated in the patient’s context to avoid misdiagnosis. The present study has a number of limitations. First, it is a single center study and the findings need to be confirmed in a larger and independent cohort. Moreover, we have only analyzed a specific group of patients (patients with severe neurological injury). The applicability of these biomarkers should be tested in different pathological contexts such as severe acute pancreatitis, trauma, burn or surgery. We found a combination of biomarkers in peripheral blood able to stratify ICU patients with high risk to develop sepsis. These results have potentially important implications in the hospital care area, as the combination of parameters studied can facilitate the management of critically ill patients.
We are grateful to Mr. M. Fernandez, from the flow cytometry IGTP Platform, for his support. We acknowledge Dr. J. Roca, from the epidemiology department of Germans Trias i Pujol University Hospital, for his help and support for statistical analysis, and the ICU nurse team from Germans Trias i Pujol for their nursing care. We also convey our gratitude to Mr. Jozef Martin M. Dijkstra for his English grammar assistance. We would like to thank editage (www.editage.com) for English language editing.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Sanchez BQ, Lucas E, Galvan OP, Argudo E, Armestar F, Caceres EM (2023) Assessment of Sepsis Index and HLA-DR Expression on Monocytes as Predictive Sepsis Biomarkers. Adv Tech Biol Med. 11:405.
Received: 23-Nov-2022, Manuscript No. ATBM-22-20372; Editor assigned: 25-Nov-2022, Pre QC No. ATBM-22-20372 (PQ); Reviewed: 09-Dec-2022, QC No. ATBM-22-20372; Revised: 22-Jun-2023, Manuscript No. ATBM-22-20372 (R); Published: 29-Jun-2023 , DOI: 10.35248/2379-1764.23.11.405
Copyright: © 2023 Sanchez BQ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.