
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2020)Volume 11, Issue 1
Objectives: Over-sedation and lingering sedation have been reported as problems associated with intravenous sedation (IVS). Saccadic eye movements can be used as parameters for the objective and quantitative evaluation of sedative effects.
Study design: Measurement of saccadic eye movements using a wearable Talk Eye Lite® (TEL) tracking device is useful for evaluating recovery following IVS. However, the assessment of awakening by measuring saccadic eye movements in subjects treated with flumazenil following midazolam sedation has not yet been reported. In the present study, the effect of flumazenil on eye movements was assessed during the recovery process.
Results: A relatively long period of time was required for the recovery of saccadic eye movements even following the injection of physiological saline. Conversely, the recovery of saccadic eye movements occurred immediately following the injection of flumazenil. Saccadic eye movement parameters reflected the recovery from induced sedation with midazolam even in cases where flumazenil was utilized. The saccadic peak velocity was delayed again 50 min after the injection of flumazenil during IVS.
Conclusion: These results suggest the usefulness of measuring saccadic eye movements using TEL for assessing the recovery of equilibrium following IVS, even in patients treated with flumazenil.
Saccadic eye movements; Midazolam; Saccadic peak velocity
Intravenous sedation (IVS) is widely used to alleviate anxiety and fear related to dental treatment and stress associated with surgical interventions. The benzodiazepine sedative, midazolam, is often used in clinical practice due to its excellent sedative and amnesic effects and pharmacological properties [1-3].
Over-sedation and lingering sedation, however, have been reported as problems associated with IVS. The benzodiazepine receptor antagonist, flumazenil, is used for counteracting these problems; however, there is a risk of falling back into a sedated state following recovery, and consciousness must be carefully evaluated because midazolam has a longer half-life than flumazenil [4-6].
Saccadic eye movements can be used as a parameter for the objective and quantitative evaluation of the sedative effect [7-12]. “Saccadic eye movements” refers to eye movements in which the gaze rapidly shifts from one target to another so as to align the targets with the fovea centralis, which has the best visual acuity.
Visual input is initially projected to the occipital lobe, and the sensory signals are subsequently converted into motor signals in the frontal lobe after reaching Brodmann area 8. Saccadic eye movements are then expressed via the brain stem, cerebellum etc. [13].
Griffiths et al., showed that saccadic eye movements are a sensitive index of the sedative effect of benzodiazepines, [14] and the measurement of saccadic eye movements made using a wearable Talk Eye Lite® (TEL Takei Scientific Instruments Co., Ltd. Japan) tracking device is a useful means of evaluating recovery following IVS.
However, to the best of our knowledge, an assessment of wakefulness by measuring eye movements following the administration of flumazenil has not been previously reported. In the present study, we assessed the effect of flumazenil on eye movements during recovery from IVS by measuring eye movements in volunteers in whom flumazenil (F group) or physiological saline (PS group) was administered following midazolam IVS.
Subjects
The subjects were 32 healthy volunteers who fully understood the details of the study; informed consent was obtained from all subjects. The age, weight, and BMI of the subjects were recorded (Table 1).
| PS group | F group | |
|---|---|---|
| Age (year) | 24.0 ± 2.8 | 25.1.0 ± 1.8 |
| Weight (kg) | 66.9 ± 10.1 | 68.6 ± 13.0 |
| BMI | 23.9 ± 2.0 | 24.7 ± 2.1 |
| Male/Female | 10/6 | 10/6 |
| (mean ± SD) | ||
Table 1: Subject characteristics.
Adaptive randomization was used for allocation to each group. The present study was approved by the Ethical Review Committee of the Nippon Dental University School of Life Dentistry at Niigata (Permission No.: ECNG-H262, date of Approval: April 11, 2016, UMIN Study ID: UMIN000020857).
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Measurement conditions
Measurements were performed in a silent room, in which the temperature was maintained at approximately 25°C and the humidity at 50%. The oral intake of water and food was prohibited for at least 4 h prior to sedation. Subjects were also instructed to sleep well on the previous day and to avoid the intake of beverages that could stimulate the central nervous system, such as coffee or black tea, from early morning on the day of measurement.
Saccadic eye movements
The equipment and research layout were based on those used in a previous study (Figures 1A and 1B). To induce saccadic eye movements, a 30-s motion picture (visual target) was displayed on a 15-inch PC monitor; a 3-cm-diameter perfect circle flashed five times at two alternate positions located on a horizontal line 20 cm apart.
Figure 1: (A) Equipment and (B) Research layout.
The monitor was positioned approximately 45 cm from the subject. The subjects were instructed to open their eyes upon receiving instructions from an evaluator, and to follow the target shown on the monitor only during eye-tracking; they were also instructed to close their eyes when measurements were not being performed.
During each measurement, the average values of the measurement items described below were obtained. “Saccadic latency” is the time until the eye movements start to track the target; “saccadic time” is the time until the line of sight first reaches the target; and “saccadic peak velocity” is the maximum speed at which the eyeball tracks the target.
Romberg’s test
Subjects were instructed to stand straight, with their eyes closed and their feet together, and to maintain their posture for 30 s; the presence or absence of body swaying was noted. The same evaluator determined the final scores (measurements were started 40 min following midazolam administration for the subjects’ safety). The Romberg’s test was scored as follows: 0, no or slight sway; 1, macroscopically apparent sway; 2, marked sway, but no instability; and 3, unstable standing posture.
Subjective and objective clinical findings
Clinical findings were assessed by the same evaluator using a scoring table created according to the method reported; scores were determined as described below.
The subjective clinical findings were scored as 0, not sleepy; 1, not sleepy but some discomfort; 2, a little sleepy; and 3, sleepy or no response.
The objective clinical findings were scored as 0, open eyes and clear responses; 1, open eyes and slow responses; 2: closed eyes and slow responses; and 3: no response.
Vital signs (blood pressure, pulse, SpO2) were monitored throughout the study.
Study measurements
Subjects were asked to wear the TEL and to assume the Fowler position; an intravenous line was subsequently established in the left forearm using a 22 G intravenous cannula. After the subject had rested for 5 min with their eyes closed prior to midazolam administration, an evaluator instructed the subject to open their eyes; the evaluator then measured the eye movements, performed the Romberg’s test, and later assessed the clinical findings (both subjective and objective).
These data were determined as baseline values. After obtaining the control values, midazolam was administered via the intravenous line at a dose of 0.05 mg/kg; flumazenil was administered intravenously at a dose of 0.004 mg/kg 30 min following midazolam administration in the F group, with an equivalent amount of physiological saline administered to the PS group.
Measurements were performed prior to midazolam administration, 10 min following administration, and at 10-minute intervals thereafter for 150 min. The obtained data were subsequently compared and evaluated (Figure 2).
Figure 2: Study measurements at 10 minutes interval.
Statistical analysis
Differences between the obtained values and the control values were analyzed statistically using a two-way repeated measures analysis of variance; to analyze the differences within a group, multiple comparisons were performed using the Bonferroni method. Clinical test data were analyzed using the Friedman test and the Mann- Whitney test.
We calculated the power of a two-way RM-ANOVA at an effect size of 0.4 (Cohen’s large effect size), an alpha error probability of 0.05, β=0.85, a total sample size of 32, and two groups using the Power analysis software (G*Power 3.1.9.2) [15]. The statistical analyses were performed using the statistical SPSS 22.0 program (IBM Co.; Armonk, NY, USA).
Vital signs
No significant differences in vital signs were observed between the two groups during the study period.
Saccadic eye movements
Saccadic latency: The saccadic latency is shown in Figure 3A. A significant difference was observed between the two groups 30 min following the administration of midazolam (p<0.05).
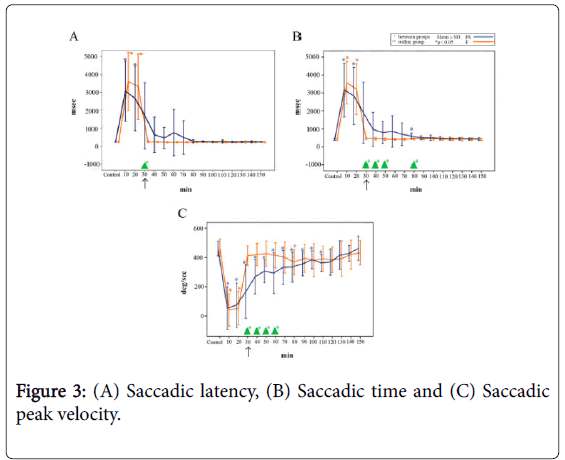
Figure 3: (A) Saccadic latency, (B) Saccadic time and (C) Saccadic peak velocity.
In the PS group, the baseline saccadic latency was 249 ± 29 ms (mean ± SD, n=16), which increased to 3064 ± 1672 ms 10 min following midazolam injection and remained significantly prolonged until 20 min after the injection (p<0.05). The saccadic latency subsequently decreased to 1693 ± 1846 ms immediately following the injection of PS, and progressively recovered thereafter, reaching 244 ± 18 ms at 150 min (i.e., 120 min after the injection of PS).
In the F group, the baseline saccadic latency was 241 ± 29 ms (mean ± SD, n=16), which increased to 3309 ± 1483 ms 10 min following midazolam injection and remained significantly prolonged until 20 min after the injection (p<0.05). The saccadic latency began to recover toward the baseline level immediately following the injection of F, ultimately reaching 254 ± 38 ms.
Saccadic time: The saccadic time is shown in Figure 3B. Significant differences were observed between the two groups from 30 min following midazolam administration until 50 min (80 min after dosing) (p<0.05). No significant differences were observed at the other time points.
In the PS group, the baseline saccadic time was 411 ± 38 ms (mean ± SD, n=16), which increased to 3162 ± 1499 ms 10 min following injection of midazolam. Immediately following the injection of PS, the saccadic time decreased to 1887 ± 1696 ms; a significant difference was observed between the baseline value and the value at 80 min after the injection of midazolam (p<0.05).
In the F group, the baseline saccadic time was 407 ± 39 ms (mean ± SD, n=16), which increased to 3570 ± 1175 ms 10 min following midazolam injection and remained significantly prolonged until 20 min after the injection (p<0.05). Immediately following the injection of F, the saccadic time recovered to 473 ± 59 ms, which was roughly equivalent to the baseline level.
Saccadic peak velocity: The saccadic peak velocity is shown in Figure 3C. Significant differences were observed between the groups from 30 min following midazolam administration until 60 min after dosing (p<0.05). The baseline value in the PS group was 482 ± 49% (mean ± SD, n=16), which decreased to 75 ± 145% 10 min following injection of midazolam.
The saccadic peak velocity was 188 ± 183% immediately following injection of PS, and a significant difference in the saccadic peak velocity was observed between the baseline value and the values measured until 110 min after the injection of midazolam (i.e., 80 min following PS injection) (p<0.05).
In the F group, the saccadic peak velocity was 464 ± 61% (mean ± SD, n=16) at baseline, which decreased to 40 ± 110 % immediately following the injection of midazolam; a significantly decreased velocity was maintained until 20 min following the injection (p<0.05).
The velocity recovered to 411 ± 67% immediately following the injection of F, which was roughly equivalent to the baseline level. At 80 min after the administration of F, the saccadic peak velocity showed a significant decrease at 367 ± 86% (p<0.05).
Romberg’s test
The results of Romberg’s test are shown in Figure 4A. Significant differences were observed between the groups from 40 min until 70 min following administration of midazolam (p<0.05).
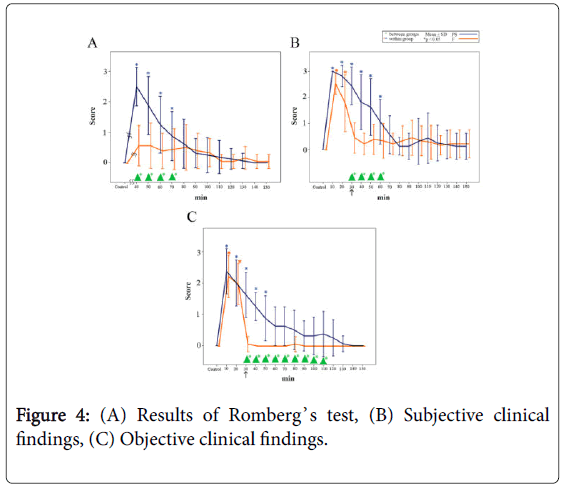
Figure 4: (A) Results of Romberg’s test, (B) Subjective clinical findings, (C) Objective clinical findings.
In the PS group, the score increased to 2.5 ± 0.6 (mean ± SD, n=16) 40 min following injection of midazolam (i.e., 10 min following the injection of PS) and remained significantly high until 70 min (i.e., 40 min following the injection of PS) (p<0.05).
In the F group, the score recovered to 0.6 ± 0.7 (mean ± SD, n=16) 40 min following injection of midazolam (i.e., 10 min following the injection of F), which was close to the baseline value.
Subjective clinical findings
The subjective clinical findings are shown in Figure 4B. Significant differences were observed between the groups from 30 min to 60 min following administration of midazolam (p<0.05).
In the PS group, the test score increased to 3.0 ± 0.1 (mean ± SD, n=16) 10 min following injection of midazolam and remained high at 2.4 ± 0.7 immediately following injection of PS; thereafter, the score gradually decreased until 60 min (i.e., 30 min following PS injection) (p<0.05).
In the F group, the test score increased from the baseline level of 0.5 ± 0.6 to 2.7 ± 0.4 (mean ± SD, n=16) 10 min following injection of midazolam and remained at a significantly higher value until 20 min (p<0.05); subsequently, the score recovered to a value close to the baseline level immediately after the injection of F.
Objective clinical findings
The objective clinical findings are shown in Figure 4C. Significant differences were observed between the groups from 30 min to 110 min following midazolam administration (p<0.05).
In the PS group, the score increased from the baseline level to 2.3 ± 0.7 (mean ± SD, n=16) 10 min following injection of midazolam, decreasing to 1.6 ± 0.7 immediately following injection of PS, but remaining significantly high until 50 min (i.e., 20 min following the injection of PS) (p<0.05).
In the F group, the score increased from the baseline level to 2.3 ± 0.7 (mean ± SD, n=16) at 10 min following injection of midazolam and remained significantly high until 20 min (p<0.05). The score subsequently decreased to 0.1 ± 0.3 immediately after the injection of F, which was close to the baseline level.
Summary of measured variables
The measured variables are summarized in Figure 5. The saccadic peak velocity in the PS group took the longest time to recover. In the F group, the saccadic latency, saccadic time, saccadic peak velocity, Romberg’s test results, and clinical findings (subjective and objective) all recovered following the administration of flumazenil. The saccadic peak velocity in the F group recovered following administration of F, but the rate decreased once again 50 min following administration of F.
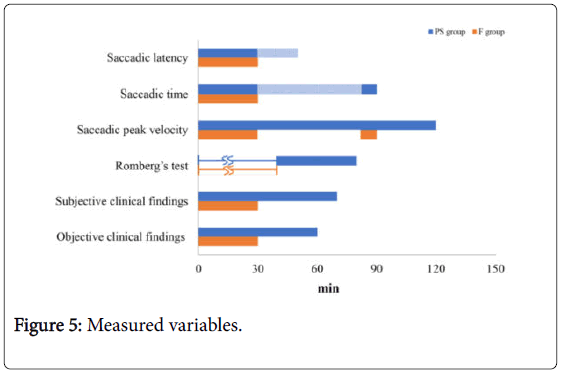
Figure 5: Measured variables.
IVS using midazolam, a benzodiazepine, may encounter such problems as intra- and/or postoperative over-sedation and a protracted sedative effect. Flumazenil, which binds to the benzodiazepine receptor and antagonizes the biological effects of benzodiazepines, by itself is devoid of any other significant pharmacological effects. Accordingly, it is used to counteract and reverse the problems associated with the use of benzodiazepines. Since the half-life of flumazenil is shorter and differs from that of midazolam, it is important to ensure cautious evaluation of the awakening state when using this antagonist, in view of the risk of the patient falling back into a re-sedated condition following recovery.
The present study was performed to examine the influence of flumazenil on saccadic eye movements and to assess the safety of evaluating awakening by measuring saccadic eye movements during recovery following IVS induced with midazolam in a group of subjects given flumazenil and a control group given physiological saline.
When examining the effect of flumazenil on saccadic eye movements in the present study, the saccadic eye movement parameters, i.e., saccadic latency, saccadic time, and saccadic peak velocity of eye movements, required a long time to recover, even in the PS group given physiological saline. Among the saccadic eye movement parameters, the recovery of the saccadic latency was evident 30 min following injection of midazolam, and the recovery of the saccadic time was evident 90 min after midazolam injection, whereas it took the saccadic peak velocity approximately 120 min to recover. The saccadic peak velocity, which took the longest time to recover, is a test parameter that is not affected by fatigue-associated changes in the measurement data and can be used as a reproducible and stable indicator. Therefore, the evaluation of the saccadic peak velocity, which takes a longer time to recover, should enable a more precise evaluation of the recovery of equilibrium following IVS.
In the F group, the recovery of saccadic eye movements, i.e., saccadic latency, saccadic time, and saccadic peak velocity, was evident immediately following injection of flumazenil. In particular, the saccadic peak velocity of the eye movements, which took a long time to recover in the PS group, recovered rapidly in response to the injection of flumazenil.
This data demonstrates that saccadic eye movement parameters reflected recovery from induced sedation with midazolam, even in cases in which flumazenil was utilized.
In addition, the subjective clinical findings in group F showed an increase in the score at 50 min and 60 min following flumazenil administration, suggestive of re-sedation. The saccadic peak velocity decreased significantly at 50 min following flumazenil administration, which was thought to have occurred as a result of re-sedation. At this time, the saccadic latency, saccadic time, Romberg’s test results, and objective clinical findings did not suggest a state of sedation. The three saccadic eye movement indicators reflected the antagonism of midazolam by flumazenil better than other traditional clinical tests, but only the saccadic peak velocity is suitable for evaluating the stage of resedation following flumazenil injection. These results suggest that the saccadic peak velocity reflects the awakening state most accurately.
Thus, the measurement of saccadic eye movement parameters is useful as an objective means of determining the awakening state following midazolam IVS in subjects injected with flumazenil, and the saccadic peak velocity was shown to objectively reflect the awakening state.
Since clinical findings (subjective and objective) and Romberg’s test can vary depending on the evaluator, accurate evaluations of the awakening state are difficult [16].
Three indicators of saccadic eye movement reflected the antagonism of midazolam by flumazenil better than other traditional clinical tests, but only the saccadic peak velocity has the potential to become a parameter for the evaluation of re-sedation following flumazenil injection. The saccadic peak velocity objectively reflected the awakening state.
We would like to express our gratitude to the doctors at the Department of Dental Anesthesiology, The Nippon Dental University School of Life Dentistry at Niigata who cooperated in conducting the research.
The authors would like to thank Enago (www.enago.jp) for the English language review.
Citation: Saitou Y, Tomita Y, Tanaka S, Sano K (2020) Assessment of Wakefulness by Measuring Eye Movements after Injection of Flumazenil during Intravenous Sedation with Midazolam. J Anesth Clin Res.11:934. DOI: 10.35248/2155-6148.20.11.934.
Received: 06-Jan-2020 Accepted: 20-Jan-2020 Published: 27-Jan-2020 , DOI: 10.35248/2155-6148.20.11.934
Copyright: © 2020 Saitou Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.