
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2024)Volume 14, Issue 5
Objective: Although many studies paid attention to the connection between dietary iron and incidence of malignant tumor. Few studies elucidated the roles dietary iron played in all-caused or cancer-caused mortality. It is paradoxical about how total dietary iron influences all-cause/cancer-cause mortality.
Methods: Our study collected dietary iron and survival data from National Health and Nutrition Examination Survey (NHANES) 1999-2020. Multivariate cox proportional risk models and subgroup analysis were used to assess the relationship between dietary iron and all/cancer-caused death. Restricted Cubic Samples (RCS) were used to access the non-linear relationship between them.
Results: Dietary iron was a protective factor against all-cause mortality (p for trend=0.004), as well as cancer-caused mortality (p for trend=0.028). They had an "L" shaped nonlinear curve between dietary iron and all-cause mortality (p for overall<0.001; p for non-linearity<0.001), so as to the cancer-related death (p for overall=0.002,p for non- linearity=0.046). With increased dietary iron, all-cause death risk decreased in those were no more than 65 years (p for trend=0.001), males (p for trend=0.02), Non-Hispanic White (p for trend=0.02), Non-Hispanic Black (p for trend<0.001), former smokers (p for trend<0.001), moderate drinkers (p for trend<0.001), heavy drinkers (p for trend<0.001), as well as without hypertension (p for trend<0.001) or DM (p for trend<0.001). Iron intake decreased cancer-caused death in individuals who were<=65 years (p for trend=0.005), males (p for trend=0.04), non-Hispanic White (p for trend=0.03) or non-Hispanic Black (p for trend=0.001), as well as never smokers (p for trend=0.002).
Conclusion: Dietary iron was a positive factor for all/cancer-caused death in population and they had an "L" shaped nonlinear relationship. All-caused or cancer-caused mortality was attenuated by dietary iron in people who were no more than 65 years, males, Non-Hispanic White and Non-Hispanic Black, as well as people without hypertension or DM.
Dietary iron; All-cause mortality; Cancer-cause Mortality; NHANES; Survival; Diet; Cox proportional hazards model
Iron is widely involved in many important processes of cell cycle, such as DNA synthesis and repair, energy metabolism and oxidation-reduction reaction [1]. The uptake, storage and recycling of iron are also essential to the maintenance of physiological cellular metabolism, which are regulated tightly and precisely. Therefore, iron has a strong oxidative potential and contribute to the development of tumor through oxidative stress [2,3]. Iron also promotes the growth of malignant cells [4]. A recent umbrella review demonstrated that increased heme iron intake was a risk factor for colorectal cancer (CRC), while total dietary iron was a protective factor against colorectal adenoma and esophageal cancer. What's more, supplementing iron could avoid these diseases [5]. The main cause of increased CRC risk in earlier study was iron and red meat instead of total dietary iron [6]. Genetically engineered mouse models with tissue-specific depletion of FBXL5 displayed more detail that iron overload in liver caused hepatocellular carcinoma (HCC) and that FBXL5 degraded iron-regulated protein 2 (IRP2) [7,8]. Relative incidence of breast cancer was increased at the highest and lowest serum iron levels [9,10]. At the same time, the serum iron increased HCC. But there is no relationship between serum iron and lung cancer [11]. Dietary iron intake affects the risk of various tumors differently.
Although there are many studies concentrate on the connection between dietary iron and tumors. Few studies elucidated the association between total dietary iron and all-cause or cancer-cause mortality. Conclusions about how total dietary iron influences all-cause/cancer-cause mortality are paradoxical. Synthetic effect of dietary vitamins and iron in males who were smokers reduced all-cause mortality and Cardiovascular Disease (CVD) mortality in the Belgian Inter-University Research on Nutrition and Health (BIRNH) study [12]. Either too low or too high dietary iron content in the Chinese Recommended Nutrient Intake (RNI) was a risk factor for death in women [13]. The NIH-AARP Diet and Health Study concluded that the overall risk of death in the population is higher when dietary intake of heme iron rose and the risk of cause-specific mortality was increased by 10% (cancer and other/ unknown causes) [11].
Apart from this, both dietary iron deprivation and iron chelator therapy inhibited the proliferation of cancer cells [14]. Recent retrospective analyses in the NHANES database concluded that there was no statistically significant association between total dietary iron and all-cause death, cardiovascular disease death, CVD and cancer-cause death [15]. However, this research included only three 2-year cycles of NHANES data. The findings were not representative of the broader American population. The NHANES survey uses a complex multistage probability sampling design. Information on health and nutritional status populations is collected through every 2-year cycles. Our study expanded the sample size by retrospectively collecting dietary iron content data and other dietary data from NHANE 1999-2020. The objective of our study was to explore the association between dietary iron and all-cause/cancer-caused mortality. This will provide a theoretical basis for dietary treatment to prevent tumor mortality.
Data source
Data in our research were collected from the NHANES database, which is available free of charge from the official NHANES website. The NHANES survey is conducted biannually by the Centers for Disease Control and Prevention (CDC) in the United States, and multistage complex sampling was used to ensure a representative sample. All enrolled populations signed informed consent.
Population
This is a retrospective study of the NHANES 1999-2020 cohort. The population exclusion criteria in our study were as follows: (1) younger than 20 years; (2) missing data on iron intake; (3) did not answer the following questions definitively: "Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?" and "What kind of cancer was it?"; (4) missing follow-up data; (5) missing data on other important covariates. Totally, 38,506 respondents were enrolled under our inclusion and exclusion criteria. The specific screening process was as follows: a total of 101,146 respondents were included in the study from 1999 to 2020, of whom 980 were younger than 20 years old, 1319 did not have data on dietary iron intake, 46,125 did not explicitly respond to the questionnaire on the prevalence of tumors, 5,121 were missing follow-up data, and 9,032 respondents were missing data on important covariates. The detailed screening flowchart is shown in Figure 1.
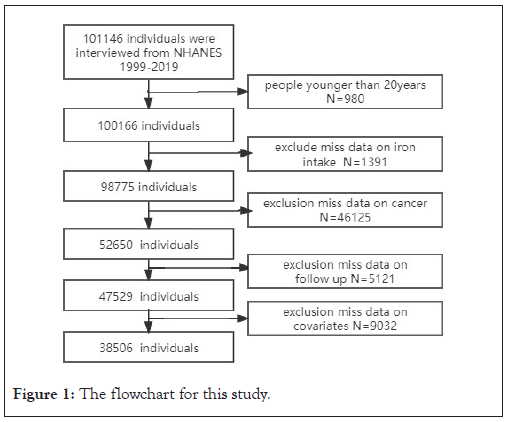
Figure 1: The flowchart for this study.
Study variables
Dietary iron intake was comprehensively assessed by trained interviewers who performed two consecutive 24-hour dietary recalls of the respondents. The first and second were collected face-to-face at the MEC test and by telephone 3-10 days later, respectively. Dietary iron intake was calculated by averaging the data from the two dietary recalls (if available), or using a single dietary recall if had data from the first interview only.
Cancer data
The "Medical Conditions" section of the NHANES interview collects information about the health status and medical history of the adult, including a history of cancer and malignancy.
Covariates
In order to create plausible cox proportional regression models as accurately as possible, potential con-founders were also included as covariates in our study, including age (<65, ≥ 65), sex (male or female), BMI, race (Non-Hispanic White, Mexican American, Other Race-including Multi-Racial, Non-Hispanic Black, and Other Hispanic), Household Poverty-to-Income Ratio (PIR), Educational Level (Some College or AA degree, 9th-11th Grade (Includes 12th grade with no Poverty Income Ratio (PIR), education level (Some College or AA degree, 9th-11th Grade (Includes 12th grade with no diploma), College Graduate or above, High School Grad/GED or Equivalent, Less Than 9th Grade), marital status (Divorced, Widowed, Married, Living with partner, Separated, Never married) and smoking history (now, former, never), alcohol consumption history (mild, moderate, former, heavy) alcohol consumption classified refer to previous published literature [16]. The criteria for diagnosis and classification of DM and hypertension were in accordance with clinical criteria.
Follow-up data
Mortality data was obtained by linking the cohort database to the National Death Index as at 31 December 2019. All-cause mortality was defined as any cause of death. We used the International Statistical Classification of Diseases and Related Health Problems (ISCDRH), with cancer-cause mortality defined according to malignant neoplasms (C00-C97).
Statistical methods
We described continuous and categorical variables as mean ± standard deviation (X ± SD) and number (percentage), respectively. All data analyses were performed using the weighting correction recommended by NHANES. Normally distributed data, non-normally distributed data and categorical variables were compared by independent samples t-test, Mann-Whitney U-test and chi-square test, respectively. We categorized dietary iron (mg) according to inter-quartile range (Q1: [0,8.97], Q2: (8.97,12.87], Q3: (12.87,18.36], Q4: (18.36,147.88]), and assessed the association between dietary iron content and all-cause risk and cancer-cause death by multivariate Cox proportional risk models that included parameters of complex sampling designs associations. Three different Cox regression models were constructed. Model 1 was not adjusted for any covariates. Model 2 was adjusted for age, sex, and race. Model 3 was further adjusted for other covariates, including PIR, education level, smoking status, alcohol use, hypertension and DM. Survival differences among various groups were compared by Kaplan-Meier analysis and log- rank test. Restricted Cubic Samples (RCS) were used to fit the non-linear relationship. Subgroup analyses were performed to explore the association among different subgroups. R (version 4.2.1) was used for statistical analyses and P<0.05 was considered statistically significant.
The clinical characteristics of individuals in 1999–2020 NHANES
A total of 38,506 individuals came from NHANES 1999-2020 were included in our study. The intake of dietary iron was (15.22 ± 0.07) mg and the mean age was (47.41 ± 0.19) years. Among them, 19370 (49.27%) were men and 19136 (50.73%) were women; and 18108 (70.94%), 6456 (7.48%), 3119 (6.11%), 7849 (10.34%), 2974 (5.13%) were Non-Hispanic White, Mexican American, Other Race-including Multi-Racial, Non-Hispanic Black, Other Hispanic, respectively. What's more, 3754(9.80%) people had cancers. When it comes to marital status, the population in group of divorced, widowed, married, living with partner, separated and never married groups were 4224(10.50%), 3244(5.74%), 20698(57.60%), 2845(7.48%), 1270(2.43%) and 6225 (16.25%), respectively. Smoking history was classified as now (8210(21.48%)), former (9909(25.42%)) and never (20368(53.08%)) according to the criteria described in the methods section. A total of 16,723 (37.76%) individuals had hypertension, 6,737 (12.86%) individuals had diabetes, and 1,693 (4.29%) individuals had IFG. We found a trend of decreasing dietary iron intake along with time (p<0.0001). In addition, education and alcohol consumption were shown in Table 1.
| Variables | Total | 1999-2000 | 2001-2002 | 2003-2004 | 2005-2006 | 2007-2008 | 2009-2010 | 2011-2012 | 2013-2014 | 2015-2016 | 2017-2018 | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iron_mg | 15.22(0.07) | 15.26(0.32) | 15.58(0.28) | 15.89(0.20) | 16.24(0.18) | 15.39(0.30) | 15.36(0.19) | 15.68(0.19) | 14.57(0.11) | 14.12(0.21) | 14.21(0.19) | < 0.0001 |
| Age | 47.41(0.19) | 45.77(0.52) | 45.95(0.48) | 47.20(0.53) | 47.16(0.78) | 47.20(0.42) | 47.93(0.52) | 48.25(0.79) | 48.21(0.40) | 48.58(0.61) | 47.20(0.79) | 0.001 |
| Sex | 0.93 | |||||||||||
| Male | 19370(49.27) | 1434(48.40) | 1917(49.98) | 1866(49.19) | 1906(49.39) | 2244(48.71) | 2308(49.39) | 2023(49.96) | 2113(49.54) | 2051(49.06) | 1508(48.82) | |
| Female | 19136(50.73) | 1420(51.60) | 1812(50.02) | 1800(50.81) | 1768(50.61) | 2244(51.29) | 2309(50.61) | 1917(50.04) | 2191(50.46) | 2094(50.94) | 1581(51.18) | |
| Race | 0.57 | |||||||||||
| Non-Hispanic White | 18108(70.94) | 1352(72.98) | 2015(73.88) | 2005(73.81) | 1912(73.90) | 2214(71.77) | 2375(71.41) | 1604(70.05) | 1989(68.84) | 1503(67.66) | 1139(65.91) | |
| Mexican American | 6456( 7.48) | 729(5.65) | 772(6.78) | 714(7.23) | 686(7.28) | 740(7.88) | 799(7.89) | 357(6.71) | 545(8.42) | 718(8.22) | 396(8.19) | |
| Other Race - Including Multi-Racial | 3119( 6.11) | 86(4.17) | 107(3.87) | 135(4.80) | 136(5.01) | 158(5.14) | 214(5.67) | 585(6.96) | 557(7.20) | 545(7.99) | 596(9.67) | |
| Non-Hispanic Black | 7849(10.34) | 505( 9.04) | 689( 9.98) | 707(10.65) | 833(10.64) | 914(10.80) | 804(10.58) | 1018(10.47) | 844(10.35) | 844(10.33) | 691(10.22) | |
| Other Hispanic | 2974( 5.13) | 182(8.15) | 146(5.49) | 105(3.52) | 107(3.18) | 462(4.42) | 425(4.45) | 376(5.82) | 369(5.19) | 535(5.80) | 267(6.02) | |
| Cancer | < 0.0001 | |||||||||||
| No | 34752(90.20) | 2605(92.55) | 3377(91.25) | 3298(90.87) | 3354(91.76) | 4031(90.80) | 4120(89.25) | 3592(90.35) | 3865(88.18) | 3710(87.88) | 2800(90.06) | |
| Yes | 3754( 9.80) | 249( 7.45) | 352( 8.75) | 368( 9.13) | 320( 8.24) | 457( 9.20) | 497(10.75) | 348( 9.65) | 439(11.82) | 435(12.12) | 289( 9.94) | |
| Marital | 0.01 | |||||||||||
| Divorced | 4224(10.50) | 266( 9.55) | 345( 9.56) | 375(10.27) | 395(10.83) | 524(10.71) | 531(10.61) | 435(11.39) | 530(11.23) | 460( 9.76) | 363(10.79) | |
| Widowed | 3244( 5.74) | 284(5.40) | 335(5.73) | 421(6.90) | 317(5.94) | 391(5.84) | 392(5.91) | 312(5.56) | 302(5.54) | 297(5.84) | 193(4.61) | |
| Married | 20698(57.60) | 1664(60.02) | 2221(61.45) | 2013(58.97) | 2040(59.23) | 2423(57.44) | 2452(57.59) | 1910(54.09) | 2284(57.67) | 2121(55.76) | 1570(54.63) | |
| Living with partner | 2845( 7.48) | 118(4.78) | 209(6.18) | 215(6.13) | 285(8.28) | 311(7.10) | 369(7.63) | 315(8.18) | 301(6.40) | 407(9.80) | 315(9.57) | |
| Separated | 1270( 2.43) | 112(3.52) | 121(2.52) | 96(2.15) | 113(2.34) | 158(2.48) | 150(2.30) | 144(2.27) | 128(2.09) | 142(2.52) | 106(2.45) | |
| Never married | 6225(16.25) | 410(16.72) | 498(14.57) | 546(15.58) | 524(13.39) | 681(16.43) | 723(15.96) | 824(18.51) | 759(17.08) | 718(16.32) | 542(17.95) | |
| BMI | 28.89(0.07) | 28.17(0.24) | 28.02(0.15) | 28.28(0.18) | 28.67(0.26) | 28.68(0.13) | 28.95(0.13) | 28.96(0.23) | 29.35(0.18) | 29.58(0.27) | 29.94(0.30) | < 0.0001 |
| PIR | 3.06(0.03) | 2.93(0.12) | 3.13(0.07) | 3.02(0.08) | 3.16(0.06) | 3.08(0.09) | 3.04(0.05) | 2.97(0.10) | 2.98(0.11) | 3.05(0.11) | 3.23(0.07) | 0.21 |
| Smoke | < 0.0001 | |||||||||||
| now | 8210(21.48) | 590(25.19) | 864(24.69) | 841(25.25) | 830(24.04) | 1022(23.04) | 1002(19.93) | 816(20.02) | 863(19.08) | 800(18.27) | 582(16.55) | |
| former | 9909(25.42) | 804(25.09) | 1011(25.49) | 1027(25.81) | 977(25.54) | 1159(24.90) | 1186(25.40) | 950(25.56) | 1058(24.95) | 1020(26.61) | 717(24.71) | |
| never | 20368(53.08) | 1457(49.71) | 1851(49.82) | 1796(48.94) | 1866(50.42) | 2306(52.06) | 2429(54.67) | 2171(54.42) | 2382(55.97) | 2320(55.13) | 1790(58.74) | |
| Alcohol user | < 0.0001 | |||||||||||
| mild | 12991(36.44) | 895(33.38) | 1279(35.39) | 1162(33.55) | 1179(34.70) | 1371(34.72) | 1504(35.82) | 1328(37.35) | 1481(36.09) | 1378(36.59) | 1414(46.91) | |
| moderate | 5818(17.39) | 390(16.91) | 511(15.79) | 497(16.13) | 546(17.04) | 644(15.70) | 682(16.81) | 588(17.02) | 686(18.35) | 633(18.25) | 641(21.87) | |
| former | 6877(14.50) | 610(16.43) | 711(16.37) | 837(18.72) | 787(17.28) | 952(17.37) | 843(15.41) | 703(14.64) | 750(14.44) | 684(13.80) | 0( 0.00) | |
| heavy | 7581(20.96) | 539(21.27) | 702(19.95) | 665(20.39) | 704(20.59) | 880(21.06) | 1041(22.32) | 794(21.28) | 791(19.53) | 793(20.15) | 672(23.44) | |
| never | 5239(10.71) | 420(12.02) | 526(12.50) | 505(11.21) | 458(10.40) | 641(11.15) | 547( 9.64) | 527( 9.71) | 596(11.58) | 657(11.21) | 362( 7.78) | |
| Hypertension | 0.06 | |||||||||||
| no | 21773(62.22) | 1599(65.20) | 2195(65.84) | 1981(61.34) | 2146(62.90) | 2498(62.54) | 2633(62.54) | 2230(61.11) | 2438(59.66) | 2324(60.78) | 1729(61.64) | |
| yes | 16723(37.76) | 1255(34.80) | 1529(34.16) | 1682(38.66) | 1527(37.10) | 1990(37.46) | 1983(37.46) | 1710(38.89) | 1866(40.34) | 1821(39.22) | 1360(38.36) | |
| DM | < 0.0001 | |||||||||||
| no | 28966(80.25) | 2337(88.10) | 3095(87.86) | 2991(86.46) | 2786(84.35) | 3151(80.30) | 3398(81.72) | 2883(81.34) | 3177(81.19) | 2878(76.17) | 2270(78.17) | |
| DM | 6737(12.86) | 397( 8.76) | 486( 8.78) | 540(10.69) | 573(11.98) | 873(14.52) | 857(14.54) | 754(14.53) | 793(15.43) | 877(16.93) | 587(14.37) | |
| IFG | 1693( 4.29) | 120(3.14) | 148(3.36) | 135(2.85) | 134(3.67) | 231(5.18) | 178(3.74) | 143(4.12) | 137(3.39) | 235(6.90) | 232(7.45) | |
| Iron_mg | < 0.0001 | |||||||||||
| Q1 | 9645(22.66) | 761(23.47) | 866(20.93) | 807(19.66) | 791(19.47) | 1103(21.25) | 1098(21.10) | 943(22.03) | 1154(25.57) | 1217(26.89) | 905(26.00) | |
| Q2 | 9613(24.74) | 716(25.18) | 944(25.51) | 901(23.31) | 900(23.64) | 1134(24.47) | 1141(24.64) | 960(23.74) | 1093(25.52) | 1041(25.80) | 783(25.71) | |
| Q3 | 9622(26.02) | 684(25.53) | 968(26.40) | 967(27.71) | 909(25.67) | 1145(26.79) | 1218(27.83) | 1001(24.99) | 1039(24.93) | 963(24.02) | 728(26.63) | |
| Q4 | 9626(26.57) | 693(25.81) | 951(27.15) | 991(29.32) | 1074(31.22) | 1106(27.49) | 1160(26.42) | 1036(29.23) | 1018(23.99) | 924(23.30) | 673(21.66) |
Note: Data were presented as mean ± SD or n (%). DM: Diabetes Mellitus; BMI: Body Mass Index; PIR: Household Poverty-to-Income Ratio; Q1: Quartile 1; Q2: Quartile 2; Q3: Quartile 3; Q4: Quartile 4.
Table 1: Baseline characteristics of included population.
The association of dietary iron intake with mortality
In model 1, dietary iron had the potential to reduce all-cause mortality in the groups of Q2 (HR=0.83(0.76-0.91), p<0.0001), Q3 (HR=0.69(0.63-0.76), p<0.0001) and Q4 (HR=0.66(0.60-0.72, p<0.0001) (p for trend<0.001) compared with Q1 group. And there was a trend toward decrease all-cause mortality in Model 2 (p for trend<0.001) when dietary iron content increased (Q2: HR=0.85 (0.78-0.92), Q3: HR=0.73 (0.66-0.81), Q4: HR=0.71 (0.63-0.78)). Furthermore, the result in Model3 was consistent with previous results, dietary iron was a protective factor against all-cause mortality (Q2: HR=0.94 (0.86-1.03), Q3: HR=0.84 (0.75-0.94), Q4: HR=0.87 (0.78-0.96), p for trend=0.004), as shown in Table 2. We also regarded dietary iron as a continuous variable, increased dietary iron intake also reduced all-cause mortality (Model1: HR=0.98 (0.98-0.99), p<0.001, Model2: HR=0.99 (0.98-0.99), p<0.001, Model3: HR=0.99 (0.99-1.00), p=0.002), see supplementary Table 1. Kaplan-Meier survival curves in Figure 2, showed a reduction in all-cause mortality with dietary iron rose (p<0.0001).
| Model1 | p for trend | Model2 | p for trend | Model3 | p for trend | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Character | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |||
| All-cause mortality | <0.001 | <0.001 | 0.004 | |||||||||
| iron_mg | ||||||||||||
| Q1 | - | - | - | - | - | - | - | - | - | |||
| Q2 | 0.83 | 0.76-0.91 | <0.001 | 0.85 | 0.78-0.92 | <0.001 | 0.94 | 0.86-1.03 | 0.16 | |||
| Q3 | 0.69 | 0.63-0.76 | <0.001 | 0.73 | 0.66-0.81 | <0.001 | 0.84 | 0.75-0.94 | 0.003 | |||
| Q4 | 0.66 | 0.60-0.72 | <0.001 | 0.71 | 0.63-0.78 | <0.001 | 0.87 | 0.78-0.96 | 0.01 | |||
| Cancer mortality | 0.005 | 0.003 | 0.028 | |||||||||
| iron_mg | ||||||||||||
| Q1 | - | - | - | - | - | - | - | - | - | |||
| Q2 | 0.85 | 0.71-1.02 | 0.08 | 0.86 | 0.71-1.04 | 0.12 | 0.89 | 0.73-1.09 | 0.27 | |||
| Q3 | 0.78 | 0.64-0.96 | 0.02 | 0.79 | 0.65-0.96 | 0.02 | 0.85 | 0.73-1.09 | 0.14 | |||
| Q4 | 0.75 | 0.61-0.91 | 0.004 | 0.73 | 0.59-0.90 | 0.003 | 0.76 | 0.73-1.09 | 0.02 | |||
| Heart disease mortality | 0.176 | <0.001 | 0.05 | |||||||||
| iron_mg | ||||||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 1.14 | 0.94-1.40 | 0.19 | 0.85 | 0.71-1.01 | 0.07 | 0.99 | 0.82-1.20 | 0.94 | |||
| Q3 | 1.02 | 0.81-1.27 | 0.88 | 0.68 | 0.57-0.80 | <0.001 | 0.79 | 0.65-0.96 | 0.02 | |||
| Q4 | 1.29 | 0.98-1.70 | 0.07 | 0.63 | 0.52-0.78 | <0.001 | 0.84 | 0.66-1.07 | 0.15 | |||
| Respiratory disease mortality | 0.09 | <0.001 | <0.001 | |||||||||
| iron_mg | ||||||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 1.55 | 1.05-2.30 | 0.03 | 0.83 | 0.61-1.15 | 0.26 | 1.03 | 0.73-1.46 | 0.87 | |||
| Q3 | 1.44 | 0.86-2.42 | 0.17 | 0.57 | 0.39-0.84 | 0.004 | 0.68 | 0.46-1.01 | 0.06 | |||
| Q4 | 1.86 | 0.98-3.53 | 0.06 | 0.4 | 0.28-0.58 | <0.001 | 0.56 | 0.39-0.81 | 0.002 | |||
| Cerebrovascular disease mortality | 0.874 | 0.034 | 0.528 | |||||||||
| iron_mg | ||||||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 0.97 | 0.62-1.51 | 0.88 | 0.94 | 0.67-1.33 | 0.73 | 0.92 | 0.62-1.38 | 0.69 | |||
| Q3 | 0.9 | 0.59-1.38 | 0.62 | 0.68 | 0.48-0.98 | 0.04 | 0.83 | 0.57-1.21 | 0.34 | |||
| Q4 | 0.98 | 0.55-1.75 | 0.95 | 0.73 | 0.50-1.06 | 0.1 | 0.9 | 0.58-1.38 | 0.62 | |||
| Influenza and pneumonia mortality | 0.765 | 0.138 | 0.547 | |||||||||
| iron_mg | ||||||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 1.08 | 0.51-2.32 | 0.84 | 0.77 | 0.41-1.45 | 0.42 | 0.93 | 0.47-1.84 | 0.83 | |||
| Q3 | 1 | 0.42-2.38 | 1 | 0.66 | 0.34-1.28 | 0.22 | 0.76 | 0.38-1.54 | 0.45 | |||
| Q4 | 1.25 | 0.42-3.69 | 0.69 | 0.58 | 0.28-1.18 | 0.13 | 0.84 | 0.40-1.75 | 0.64 | |||
| DM mortality | ||||||||||||
| iron_mg | 0.446 | 0.531 | 0.298 | |||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 0.9 | 0.51- 1.59 | 0.71 | 1.05 | 0.64-1.71 | 0.85 | 0.89 | 0.50- 1.56 | 0.67 | |||
| Q3 | 0.67 | 0.36- 1.24 | 0.2 | 0.59 | 0.37-0.96 | 0.03 | 0.67 | 0.36- 1.23 | 0.19 | |||
| Q4 | 1.52 | 0.74- 3.11 | 0.25 | 0.96 | 0.57-1.62 | 0.89 | 1.46 | 0.84- 2.54 | 0.18 | |||
| Kidney disease mortality | 0.258 | 0.58 | 0.881 | |||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 0.53 | 0.30-0.94 | 0.03 | 0.56 | 0.32-0.99 | 0.04 | 0.67 | 0.36-1.23 | 0.2 | |||
| Q3 | 0.5 | 0.30-0.85 | 0.01 | 0.56 | 0.33-0.95 | 0.03 | 0.69 | 0.40-1.18 | 0.18 | |||
| Q4 | 0.72 | 0.46-1.14 | 0.16 | 0.84 | 0.52-1.37 | 0.49 | 1.05 | 0.58-1.89 | 0.88 | |||
| Alzheimer mortality | 0.63 | 0.63 | 0.25 | |||||||||
| Q1 | - | - | - | - | - | - | ||||||
| Q2 | 0.82 | 0.54-1.26 | 0.37 | 0.94 | 0.62-1.42 | 0.77 | - | 0.96 | 0.62-1.49 | 0.84 | ||
| Q3 | 0.95 | 0.60-1.49 | 0.81 | 1.17 | 0.74-1.86 | 0.49 | 1.19 | 0.71-2.00 | 0.5 | |||
| Q4 | 0.84 | 0.54-1.32 | 0.46 | 1.25 | 0.78-1.99 | 0.36 | 1.45 | 0.86-2.43 | 0.16 | |||
Note: a: Data were adjusted for NHANES survey weights; Model 1 was not adjusted for any covariates; Model 2 was adjusted for age, sex, and race; Model 3 was further adjusted for other covariates, including PIR, education level, smoking status, alcohol use, hypertension and DM; HR: Hazard Ratio; CI: Confidence Interval.
Table 2: Relationship between dietary iron intake and risk of mortality in different modelsa.
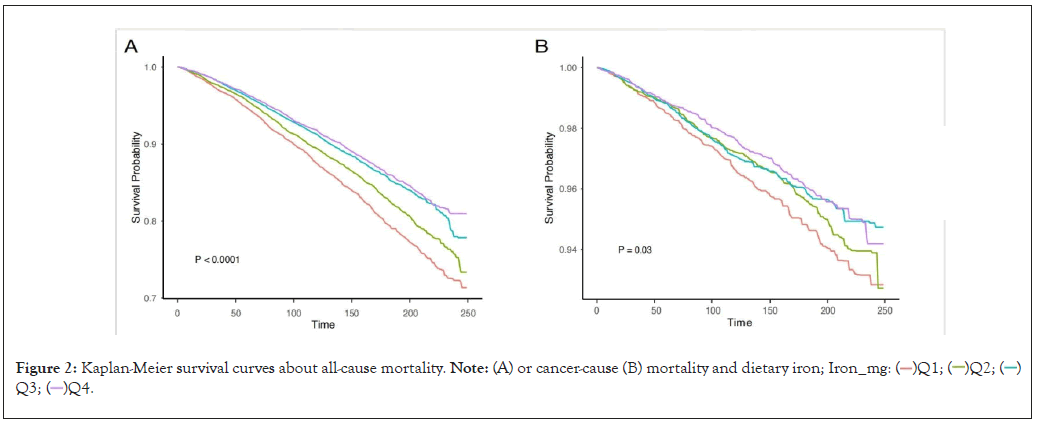
Figure 2: Kaplan-Meier survival curves about all-cause mortality. Note: (A) or cancer-cause (B) mortality and dietary iron; Iron_mg:  Q3;
Q3;  Q4.
Q4.
When it came to the relationship between dietary iron and cancer-cause mortality, we constructed three regression models and all of the models showed a reduced cancer-cause mortality trend (Table 2). P for trend in Model1 was 0.005 and HRs for Q2, Q3 and Q4 were 0.85 (0.71-1.02), 0.78 (0.64-0.96) and 0.75 (0.61-0.91). P for trend in Model2 was 0.003 (Q2: HR= 0.86 (0.71-1.04, Q3: HR= 0.79 (0.65-0.96), Q3: HR= 0.73 (0.59-0.91)). in Model3 the p for trend was 0.028 (Q2: HR=0.89(0.73-1.09), Q3: HR=0.89(0.73-1.09), Q4: HR=0.89 (0.73-1.09)). When the dietary iron content was treated as a continuous variable to construct the survival model, the results also suggested that dietary iron reduced the risk of cancer-cause death (Model1: HR= 0.99(0.98,1.00), p=0.01, Model2: HR=0.99(0.98,1.00), p=0.01 and Model3: HR= 0.99(0.98,1.00), p=0.02), see supplementary Table 1. The Kaplan-Meier curves are shown in Figure 2B.
The associations between dietary iron and mortality in different subgroups
To examine the relationship between dietary iron and all/cancer- cause death in various subgroups of age, sex, hypertension and diabetes, we adjusted the covariates of age, sex, income, education level, smoke, drink status, hypertension and diabetes. There was a tendency for all-cause death risk to decrease with increased dietary iron intake in those aged no more than 65 years (Q2: HR=0.86 (0.74-1.01), Q3: HR=0.75 (0.64-0.88), Q4: HR=0.76 (0.64-0.91), p for trend=0.001). This kind of trend was also present in males (Q2: HR=0.94 (0.80-1.09), Q3: HR=0.85 (0.73-0.99), Q4: HR=0.83 (0.71-0.98), p for trend=0.02), whereas dietary iron had no influence in females (p for trend=0.10). As for race, death risk had a trend to declining among whose race was Non-Hispanic White (p for trend=0.02) and Non-Hispanic Black (p for trend<0.001). In addition, dietary iron also attenuated the risk of all-cause mortality in population who were former smokers (p for trend<0.001), moderate drinkers (p for trend<0.001), heavy drinkers (p for trend<0.001), as well as without hypertension (p for trend<0.001) or DM (p for trend<0.001) (Table 3).
| Characters | Q1 | Q2 | p | Q3 | p | Q4 | p | p for trend |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| <= 65 years | - | 0.86(0.74-1.01) | 0.07 | 0.75(0.64-0.88) | <0.001 | 0.76(0.64-0.91) | 0.002 | 0.001 |
| >65 years | - | 0.98(0.86-1.11) | 0.75 | 0.90(0.78-1.05) | 0.17 | 0.94(0.83-1.08) | 0.4 | 0.27 |
| Sex | ||||||||
| Male | - | 0.94(0.80-1.09) | 0.4 | 0.85(0.73-0.99) | 0.03 | 0.83(0.71-0.98) | 0.03 | 0.02 |
| Female | - | 0.94(0.82-1.06) | 0.3 | 0.81(0.69-0.96) | 0.02 | 0.93(0.79-1.10) | 0.4 | 0.1 |
| Race | ||||||||
| Non-Hispanic White | - | 0.91(0.81-1.02) | 0.11 | 0.83(0.72-0.96) | 0.01 | 0.85(0.75-0.97) | 0.02 | 0.02 |
| Mexican American | - | 1.00(0.75-1.35) | 0.99 | 1.05(0.77-1.43) | 0.78 | 0.80(0.59-1.07) | 0.14 | 0.19 |
| Other Race - Including Multi-Racial | - | 1.86(1.12-3.09) | 0.02 | 1.54(0.85-2.79) | 0.16 | 1.19(0.64-2.22) | 0.57 | 0.91 |
| Non-Hispanic Black | - | 0.92(0.75-1.12) | 0.41 | 0.72(0.60-0.87) | <0.001 | 0.73(0.57-0.93) | 0.01 | <0.001 |
| Other Hispanic | - | 0.71(0.41-1.22) | 0.21 | 0.78(0.48-1.28) | 0.33 | 1.15(0.65-2.06) | 0.63 | 0.67 |
| Smoke | ||||||||
| Now | - | 1.05(0.88-1.26) | 0.58 | 0.93(0.75-1.16) | 0.53 | 0.82(0.64-1.07) | 0.14 | 0.12 |
| Former | - | 0.85(0.74-0.99) | 0.04 | 0.80(0.67-0.96) | 0.02 | 0.82(0.70-0.97) | 0.02 | 0.03 |
| Never | - | 0.96(0.82-1.11) | 0.57 | 0.79(0.66-0.95) | 0.01 | 0.91(0.77-1.07) | 0.26 | 0.08 |
| Alcohol user | ||||||||
| Mild | - | 0.97(0.82-1.15) | 0.75 | 0.86(0.71-1.04) | 0.12 | 0.90(0.75-1.08) | 0.24 | 0.16 |
| Moderate | - | 0.70(0.53-0.92) | 0.01 | 0.48(0.35-0.67) | <0.0001 | 0.53(0.39-0.72) | <0.0001 | <0.0001 |
| Former | - | 0.87(0.73-1.03) | 0.11 | 0.93(0.78-1.12) | 0.45 | 0.85(0.69-1.04) | 0.11 | 0.2 |
| Heavy | - | 1.02(0.72-1.45) | 0.9 | 0.79(0.52-1.18) | 0.24 | 0.71(0.52-0.96) | 0.02 | 0.01 |
| Never | - | 1.13(0.90-1.43) | 0.3 | 0.81(0.63-1.04) | 0.09 | 1.24(0.97-1.59) | 0.09 | 0.54 |
| Hypertension | ||||||||
| No | - | 0.82(0.68-1.00) | 0.05 | 0.74(0.60-0.90) | 0.003 | 0.73(0.60-0.90) | 0.003 | 0.003 |
| Yes | - | 0.97(0.87-1.08) | 0.55 | 0.87(0.76-1.00) | 0.05 | 0.91(0.81-1.03) | 0.13 | 0.06 |
| DM | ||||||||
| No | - | 0.97(0.86-1.10) | 0.61 | 0.85(0.74-0.98) | 0.03 | 0.83(0.72-0.97) | 0.02 | 0.01 |
| DM | - | 0.93(0.78-1.11) | 0.42 | 0.87(0.70-1.08) | 0.2 | 1.02(0.85-1.22) | 0.83 | 0.95 |
| IFG | - | 0.72(0.48-1.08) | 0.11 | 0.77(0.54-1.08) | 0.13 | 0.71(0.49-1.02) | 0.07 | 0.1 |
Note: IFG: Impaired Fasting Glucose; a: Data were adjusted for NHANES survey weights
Table 3: The association between dietary iron intakes with all-cause mortality in different subgroupsa
Iron intake decreased death caused by cancer in individuals who were<=65 years (p for trend=0.005), males (p for trend=0.04), non- Hispanic White (p for trend=0.03) or non-Hispanic Black (p for trend=0.001), as well as never smokers (p for trend=0.002). Dietary iron had a trend to reduce cancer-cause mortality in patients without hypertension (p for trend=0.05) and DM (p for trend=0.01) (Supplementary Table 2).
The nonlinear relationship between iron intake and mortality
We explored whether there was a nonlinear relationship between dietary iron content and death through RCS analysis. Dietary iron content had a nonlinear relationship with and all-cause mortality (p for overall<0.001; p for non-linearity<0.001) (Figure 3A). There was an "L" curve between them and the inflection point was at 22 mg, so as the risk of cancer-related death (p for overall=0.002, p for non-linearity=0.046) (Figure 3B). Dietary iron content was non-linearly associated with all-cause mortality in people younger than 65 years with an "L"-shaped curve (p for overall<0.001; p for non-linearity<0.001, the inflection point was at 22 mg) (Figure 3C). In addition, both males (p for overall<0.001; p for non-linearity<0.001) and females (p for overall<0.001; p for non-linearity<0.001) (Figure 3D), no matter with hypertension (p for overall<0.001, p for non-linearity<0.001) or not (p for overall<0.001, p for non- linearity=0.0012) (Figure 3E), the nonlinear relationship still remained. Exception of the IFG population (p for overall<0.001, p for non-linearity=0.366), there was a non-linear relationship between dietary iron and all-cause mortality in both those with DM (p for overall<0.001, p for non-linearity=0.007) or without DM (p for overall<0.001, p for non-linearity<0.001) (Figure 3F).
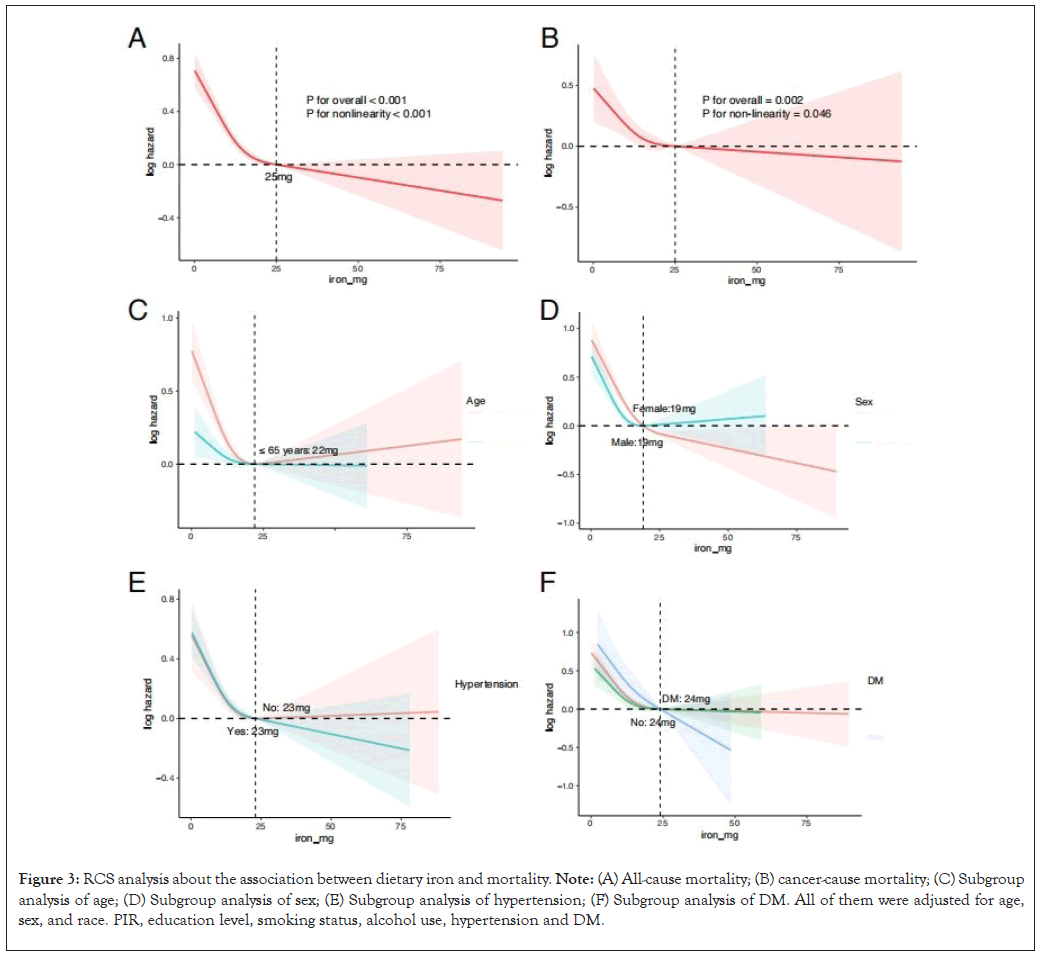
Figure 3: RCS analysis about the association between dietary iron and mortality. Note: (A) All-cause mortality; (B) cancer-cause mortality; (C) Subgroup analysis of age; (D) Subgroup analysis of sex; (E) Subgroup analysis of hypertension; (F) Subgroup analysis of DM. All of them were adjusted for age, sex, and race. PIR, education level, smoking status, alcohol use, hypertension and DM.
The nonlinear association between dietary iron with cancer-cause mortality differed from all-cause mortality in subgroups. There was a nonlinear association between dietary iron and cancer-cause death in populations who were younger than 65 years ((P for overall=0.0019, p for non-linearity=0.0165), males (P for overall=0, p for non-linearity=0.0018) and without DM (P for overall=0.0045, p for non-linearity=0.0241) (Supplementary Figure 1).
We retrospectively analyzed the association between dietary iron and all-cause or cancer-cause mortality in people from 1999-2020 in NHANES. Dietary iron reduced all/cancer-cause mortality among those were no more than 65 years, males, Non-Hispanic White and Non-Hispanic Black, and those without hypertension and diabetes. The association between dietary iron and all/cancer-cause death varied by smoke and alcohol consumption. In addition, there was a non-linear relationship between dietary iron and all/cancer-cause mortality with an 'L' shaped curve. The mentioned non-linear association was present in those who were aged<=65 years, with DM and IFG. In contrast, there was a non-linear relationship between dietary iron and death of cancer among those younger than 65 years, males and those without DM.
With the continuous development of iron metabolism and ferroptosis in biomedical field, iron played an important role in promoting tumor development and regulating immune function. Excessive iron induced cancer, such as CRC [17,18], HCC [10], prostate cancer [19], ovarian cancer [20], and breast cancer [21]. Dietary iron cooperated with inflammation in the colon collaborative activated IL-6/IL-11-Stat3 signal and developed CRC subsequently [22]. Furthermore, elevated intracellular iron levels caused malignancy by breaking cell cycle proteins and assisting JAK-STAT3 signal pathway [17]. Although dietary iron had been reported made tumor incidence to ascend in previous studies, it was interesting that our results demonstrated higher dietary iron reduced all-cause mortality and cancer-cause mortality. Malignant cells require large amounts of iron to maintain their proliferation, migration and invasion. Iron over assembled in neoplastic cells could induce death by ferroptosis [23]. Many medicines are clinically available to trigger ferroptosis and inhibit growth of tumor, such as sorafenib, artemisinin and so on [24,25]. Apart from this, CD8+ T cells were activated to perform anti-tumor functions when malignant cells occurred ferroptosis [26-28]. However, ferroptosis suppresses immune function in tumor microenvironment, thereby benefited the development of cancer [29]. Iron supplementation increases the effectiveness of anti-androgen therapy for prostate cancer in animal models [30]. Nevertheless, higher dietary iron suppresses the function of T cell and attenuates the efficacy of immunotherapy and chemotherapy in breast cancer [31]. Iron had distinct effects on immune function in different tumors, which required us to explore the relationships and links among them in detail.
The effect of dietary iron on all/cancer-cause mortality also varied in different subgroups. Dietary iron decreases the risk of death in populations aged>= 65 years or those were males, suggested that dietary iron might have an influence on sex hormone, which needed to be verified with further clinical or experimental data. The effect of dietary iron on mortality also varied across races, as previous results on dietary iron in influencing all-cause mortality in Belgian and Chinese populations have yielded different conclusions [12,13]. To our knowledge, this is the first description of an 'L'-shaped non-linear relationship between dietary iron and all-cause/cancer mortality. Dietary iron significantly reduced cancer-cause mortality until less than 25 mg, which provided a new basis for clinical dietary nutrition despite previous indications of a recommended daily intake of dietary iron [32]. Therefore, the basis for dietary iron intake may change for different purposes, such as the prevention of cancer and the improvement of survival. These differences also deserved to be further explored across subgroups of age, gender and race.
Base on a large sample from the NHANES database, we explored the relationship between dietary iron and all-cause and cancer- cause mortality through three models in categorical variables and continuous variables, respectively. The relationship was explored in different aspects. Even though, there were some limitations in our study. Firstly, the cross-sectional design of this study made it difficult to infer the causal relationship between dietary iron and all/cancer-cause death. Secondly, the dietary iron interviews based on population might not fully reflect their actual intake levels. Thirdly, despite controlled the covariates, our study still has unmeasured factors as well as measurement error, which may mislead our results. The question about how dietary iron affects prognosis in patients with cancer was not further analyzed because of the lack of medication history, tumor stage, surgery history and the differentiation, which is important to survival. In the future we need more data and detailed standard measures of dietary iron to assess its impact on death risk.
In conclusion, our study found that dietary iron was a protective factor for death of all-cause and cancer in population and they had an "L" shaped nonlinear relationship. All-cause/cancer mortality was attenuated by dietary iron in people who were aged<=65 years, males, Non-Hispanic White and Non-Hispanic Black, as well as people without hypertension or DM.
This work did not receive any specific grant from funding agencies in the public or commercial.
We would particularly like to acknowledge the staff members of NHANES, for their wonderful data support.
Supplementary data associated with this article were shown at the bottom of the article.
Jiahong Yi: Conceptualization, Methodology, Manuscript writing;
Hui Guo: Conceptualization, Formal analysis, Visualization;
Junyi Duan: Data curation, Methodology, Software;
Chang Jiang: Ju Xue, Yue Zhao: Software; Visualization;
Wenzhuo He: Data curation, Writing–review and editing, Validation;
Liangping Xia: Data curation, Conceptualization, Validation.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yi J, Guo H, Yang L, Jiang C, Duan J, Xue J, et al (2024) Association between Dietary Iron Levels and All-Cause and Cancer-Cause Mortality: A Prospective Cohort Study. J Clin Trials. 14:568.
Received: 24-May-2024, Manuscript No. JCTR-24-31718; Editor assigned: 27-May-2024, Pre QC No. JCTR-24-31718(PQ); Reviewed: 10-Jun-2024, QC No. JCTR-24-31718; Revised: 17-Jun-2024, Manuscript No. JCTR-24-31718(R); Published: 24-Jun-2024 , DOI: 10.35248/2167-0870.24.14.568
Copyright: © 2024 Yi J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.