International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
+44 1300 500008
ISSN: 2329-9096
+44 1300 500008
Research Article - (2022)Volume 10, Issue 6
Background: The ability to turn over is thought to reflect trunk function, and trunk function has been associated with the prognosis of stroke. Here, we evaluated the relationship between the ability to turn on admission and bedridden state at discharge in stroke patients.
Methods and findings: The study was conducted as a retrospective cohort study in a major Japanese regional hospital. Consecutive patients admitted between April 2018 to March 2019 with a diagnosis of “cerebral infarction” or “cerebral hemorrhage” were included. The definition of ‘turning over impossible’ was a listing for the need for partial or total assistance among the basic movement items recorded in the comprehensive rehabilitation implementation plan. Primary outcome was a bedridden status at discharge, defined as a score of 5 points on the modified Rankin Scale. The association between the early ability to turn over on admission and a bedridden state at discharge was analyzed by a logistic regression model with adjustment for gender, age, pre-hospitalization mRS, and presence of paralysis at admission as potential confounders. Among 1317 patients admitted, 448 patients met the study criteria. Of the 448 subjects, 254 were male, mean age was 76.1 (12.3) years and mean length of hospital stay was 27.4 (16.7) days. Odds ratio for a classification of “turning movements impossible” was 5.6 (95% confidence interval (CI) 2.3-13.9, p<0.01) and C-statistic was 0.82 (95% CI 0.77-0.87).
Conclusion: We found a statistically significant association between turning movements and a bedridden status in acute stroke patients.
Stroke; Rehabilitation; Prediction
The incidence of stroke is increasing and affects one in four people worldwide, surpassing the estimated rate of one in six people for dementia [1,2]. Deaths from stroke in Japan number about 130,000 a year. Stroke is also the leading cause of becoming bedridden [3]. A national survey by the Japanese Ministry of Health, Labor and Welfare in 2016 identified stroke as the second-most common cause of a need for nursing care, following dementia. Among respondents, 73.6% of men and 76.8% of women reported that ‘family illness and caregiving to ill family members’ were the main causes of caregiver worry and stress [4]. These findings highlight the need for appropriate social measures for acute stroke patients, consistent with their level of physical function at the time of discharge, to avoid an excessive burden on both patients themselves and their caregivers. The ability to predict at the time of hospitalization the level of care that patients will require at discharge and their subsequent physical progress would therefore likely aid in adjusting their social environment at the earliest possible time.
Several reports have investigated predictors of outcome in acute stroke patients, including age and gender [5], consciousness level [6,7], modified National Institutes of Health Stroke Scale (mNIHSS) [8], paralysis level [9], sitting and standing [9,10], premorbid dependence [11], functional independence measures [12], the Barthel Index and modified Rankin Scale (mRS) [13],hypertension [14], hyperlipidemia [15], diabetes mellitus [16], renal dysfunction [17], dementia [18], dysphagia [19], pneumonia [20], unilateral spatial neglect [21], and D-dimer [22] and C-Reactive Protein (CRP) levels [23]. However, few studies have investigated prognostic factors that are predictive early in the course of stroke, and new predictors are urgently required.
We focused on "turning over," which includes trunk rotation, as a prognostic factor that can be evaluated and subject to intervention from the initiation of acute stroke rehabilitation. Using the Postural Assessment Scale for Stroke Patients (PASS), Benaim, et al. reported that roughly 30% of patients could not turn onto the affected side and roughly 40% could not turn onto the nonaffected side on Day 30 [24], demonstrating the importance of early assessment after stroke. Huang, et al. evaluated the prognosis of gait, and reported that the initial static PASS score, dynamic PASS score, and turning over were predictors for independent walking in stroke patients after rehabilitation. The multivariate analysis in that study did not sufficiently adjust for explanatory variables [25] and it is not clear whether turning over was an independent factor. Overall, few reports have evaluated turning over or verified the prediction of functional prognosis based on turning over alone, and further verification is necessary.
Here, we examined the relationship between the ability to turn over at the start of in-hospital rehabilitation and bedridden state at discharge in patients hospitalized for acute cerebrovascular disease.
Patients and study design
We conducted a retrospective cohort study in a major Japanese regional hospital serving a population of approximately 370,000. The hospital is a tertiary teaching hospital with 1048 beds. Consecutive patients admitted to the Departments of Neurology and Neurosurgery with a diagnosis of “cerebral infarction” or “cerebral hemorrhage” between April 2018 to March 2019 was included. Patients with neurodegenerative disease, brain tumor, traumatic disease, subarachnoid hemorrhage, post-operative cerebral infarction and cerebral hemorrhage, other neurological diseases, a pre-hospitalization modified Rankin Scale (mRS) score of 5, or a pre-hospitalization Glasgow Coma Scale (GCS) score of E-1 were excluded. This retrospective cohort study had not been conceived at the time the patients underwent rehabilitation, and the therapists who conducted the patient assessments were accordingly blinded to it. The analysis of this study was conducted according to a pre-designed research protocol, and no arbitrary changes were made to the analytical methods.
The study was conducted with the approval of the ethics committee of Aso Iizuka Hospital (approval no. 20029), and in accordance with the Declaration of Helsinki. In Japan, retrospective cohort studies are permitted to provide opt-out consent if it is not necessary to obtain new human materials, and if it is difficult to obtain consent from individuals or the research is of high social importance. The purpose and methods of the study were sufficiently disclosed in advance in an opt-out fashion, and consent to the present study was accordingly obtained. The study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [26]. The authors have no conflicts of interest to declare.
Exposure of interest
We set “turning over” as the exposure of interest. Turning over was defined as the ability to turn from a supine to lateral recumbent position. Data on whether or not the patient could turn over was retrospectively extracted from the basic movement items in the comprehensive rehabilitation implementation plan created at the time of the initiation of rehabilitation. Turning over was classified among basic movement items into three categories, namely independent, partial assistance and total assistance. We defined “turning over possible” for those described as independent, and “turning over impossible” for the partial assistance or total assistance stage. In addition, extraction from the comprehensive rehabilitation implementation plan was made without regard to whether turning over was toward the paralyzed or non-paralyzed side, and this variable could accordingly not be determined. The comprehensive rehabilitation implementation plan was designated by the Ministry of Health, Labor and Welfare, and its evaluation method was created by collaboration among nationally certified physical therapists, occupational therapists, speech therapists, nurses, medical social workers, and nutritionists. For the individual patient, each plan is approved by a rehabilitation doctor. For plans used in the present study, the evaluators were physical therapists employed by our rehabilitation department who have been licensed to practice physical therapy in Japan for 1 to 20 years or more.
Outcome
Primary outcome was bedridden status at discharge, defined as a mRS score of 5 points. Extraction of this outcome was by retrospective scoring using rehabilitation records and comprehensive practice plans by staff with at least 5 years of physical therapy experience.
Other measurements
Other measurements were assessed from the day of admission to the first intervention in rehabilitation, including mNIHSS at admission, presence of paralysis, pre-hospital mRS, initial mRS, presence of sensory deficits, pulse rate, Glasgow Coma Scale, diabetes mellitus, atrial fibrillation, renal dysfunction, unilateral spatial neglect, and D-dimer and C-reactive protein levels. Basic patient information was obtained at the time of admission, and laboratory data was extracted retrospectively from each patient's medical record within 24 hours of admission. Pre-admission and initial mRS was obtained in the same manner as for the primary outcome.
Statistical analysis
First, we performed descriptive statistics on baseline characteristics. Continuous variables were summarized by mean (SD), and categorical variables by real numbers and percentages. Second, we analyzed the crude relationship between the possibility of turning over at the initiation of rehabilitation and bedridden status at the time of discharge using logistic regression analysis. Next, we conducted a logistic regression analysis adjusted for potential confounders for turning over at the start of rehabilitation and bedridden at the time of discharge. Potential confounders were gender, age, pre-hospitalization mRS, and presence of paralysis at admission, as identified from previous studies [5,9,11].
Sample size was calculated from the rule of sum as 50 people as a group with 10 times the outcome of the explanatory variable, with the incidence of bedridden being 10%.
When the rate of missing variables was less than 10% [27], missing variables were imputed using the Multivariate Imputation by Chained Equations (MICE) method. Sensitivity analyses were performed by age, gender, and pre-hospital mRS, with age stratified as less than 70 years, 70-84 years, and 85 years or older, and prehospital mRS as mRS:0,1,2 and mRS:3,4. Statistical significance was set at less than 5%. All statistical analyses were performed using Stata version 15.1 (Stata Corp, College Station, TX, USA). Statistical methods were described using logistic regression analysis with univariate and multivariate analyses after summarizing the information at admission and logistic regression analysis with stratified analysis for sensitivity analysis. Significance level was set at less than 5%.
Baseline characteristics of patients
Among 1317 patients admitted to the Departments of Neurology and Neurosurgery of our hospital from April 2018 to March 2019, 477 patients with cerebral infarction or cerebral hemorrhage were selected after exclusion of those with neurological intractable disease (n=130), brain tumor (n=59), traumatic disease (n=215), subarachnoid hemorrhage (n=141), postoperative cerebral infarction or cerebral hemorrhage (n=90), and other diseases (n=205). Of these 477 patients, 29 with a pre-hospital mRS score of 5 (n=16) and pre-hospital GCS Eye opening score of 1 (n=13) were excluded. Subject recruitment is shown in Figure 1 and basic characteristics are listed in Tables 1 and 2.
| Variable | Total (n=448) | Turning over possible (n=213) | Turning over impossible (n=235) | Missing |
|---|---|---|---|---|
| Sex | 0 | |||
| Male | 254 (56.6%) | 133 (62.4%) | 121 (51.49%) | - |
| Female | 194 (43.3%) | 80 (37.6%) | 114 (48.51%) | - |
| Age (years) | 76.1 (12.3) | 73.0 (12.4) | 78.8 (11.6) | 0 |
| Length of hospital stay (days) | 27.4 (16.7) | 21.6 (12.6) | 32.7 (18.1) | 0 |
| Type of Stroke | 0 | |||
| Ischemic | 416 (92.8%) | 207 (97.1%) | 209 (88.9%) | - |
| Hemorrhagic | 32 (7.1%) | 6 (2.4%) | 26 (11.6%) | - |
| Pre-hospitalization mRS (Score) | 0 | |||
| 0 | 302 (67.4%) | 179 (84.0%) | 123 (52.3%) | 0 |
| 1 | 32 (7.1%) | 8 (3.8%) | 24 (10.2%) | 0 |
| 2 | 21 (4.6%) | 5 (2.4%) | 16 (6.8%) | 0 |
| 3 | 21 (4.6%) | 5 (2.4%) | 16 (6.8%) | 0 |
| 4 | 72 (16.0%) | 16 (7.51%) | 56 (23.8%) | 0 |
| Initial mRS (Score) | ||||
| 2 | 14 (3.1%) | 14 (6.6%) | 0 (0%) | |
| 3 | 63 (14.1%) | 56 (26.3%) | 7 (3.0%) | |
| 4 | 200 (44.6%) | 134 (62.9%) | 66 (28%) | |
| 5 | 171 (38.2%) | 9 (4.2%) | 162 (68.9%) | |
| mRS (score) at discharge | 0 | |||
| 0 (5-6) | 53 (11.9%) | 6 (2.8%) | 47 (20%) | - |
| 1 (0-4) | 395 (88.1%) | 207 (97.1%) | 188 (80%) | - |
| Glasgow coma scale (Eye opening) | ||||
| 2 | 9 (2.0%) | 0 (0%) | 9 (3.8%) | 0 |
| 3 | 38 (8.5%) | 7 (3.3%) | 31 (13.2%) | 0 |
| 4 | 401 (89.5%) | 206 (96.7%) | 195 (83%) | 0 |
| Paretic side | 0 | |||
| Right | 219 (48.8%) | 108 (50.7%) | 111 (47.2%) | - |
| Left | 196 (37.7%) | 80 (37.6%) | 116 (49.4%) | - |
| Paralysis | 414 (92.4%) | 188 (88.3%) | 226 (96.1%) | 0 |
| Sensory impairment | 118 (26.3%) | 48 (22.5%) | 70 (29.8%) | 0 |
| DM | 111 (24.7%) | 55 (25.8%) | 56 (23.8%) | 0 |
| Cerebrovascular accident | 133 (29.6%) | 51 (23.9%) | 82 (34.9%) | 0 |
| Dementia | 65 (14.5%) | 13 (6.1%) | 52 (22.1%) | 1 |
| mNIHSS | 7.1 (8.4) | 2.5 (3.7) | 11.3 (9.2) | |
| USN | 46 (10.2%) | 13 (6.2%) | 33 (14%) | 2 |
| Af | 64 (14.2%) | 30 (14.1%) | 34 (14.5%) | 0 |
| Renal function impairment | 35 (7.8%) | 16 (7.5%) | 19 (8.0%) | 0 |
| D-dimer (ug/mL) | 2.7 (5.2) | 1.8 (4.8) | 3.5 (5.4) | 24 |
| C-reactive protein (mg/dL) | 0.9 (2.5) | 0.5 (1.5) | 1.3 (3.2) | 0 |
| HR (/min) | 75.8 (14.2) | 74.7 (14.1) | 76.8 (14.4) | 0 |
| BMI (kg/m2) | 22.4 (4.0) | 23.0 (3.5) | 21.8 (4.3) | 1 |
| Sitting start days (days) | 1.6 (1.1) | 1.2 (0.5) | 1.9 (1.4) | 5 |
| Standing start days (days) | 3.4 (3.7) | 1.9 (1.5) | 4.9 (4.5) | 16 |
| Walking start days (days) | 5.2 (6.8) | 2.7 (3.5) | 8.2 (8.4) | 60 |
Note: mRS: modified Rankin Scale; DM: Diabetes Mellitus; USN: Unilateral Spatial Neglect; Af: Atrial fibrillation; HR: Heart Rate; BMI: Body Mass Index; mNIHSS: modified National Institutes of Health Stroke Scale
Table 1: Baseline characteristics of patients.
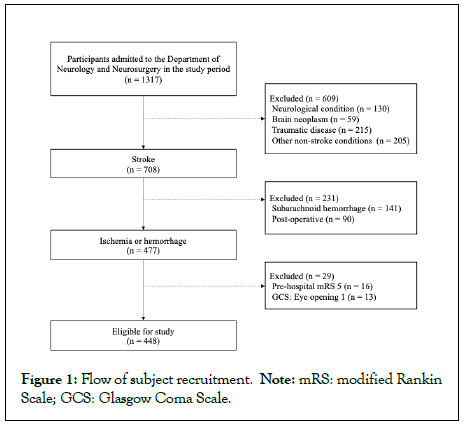
Figure 1: Flow of subject recruitment. Note: mRS: modified Rankin Scale; GCS: Glasgow Coma Scale.
Of the 448 subjects, 254 were male and 194 were female. Mean age was 76.1 (12.3) years (SD) and mean length of hospital stay was 27.4 (16.7) days. 235 patients could not turn over at the start the rehabilitation while 213 patients could. 53 patients were bedridden at discharge.
Of the patients who could turn over, 207 had cerebral infarction and 6 had cerebral hemorrhage; 133 were male and 80 were female; mean age was 73.0 (12.4) years; and mean hospital stay was 21.6 (12.6) days. Of the patients who could not turn over, 209 patients had cerebral infarction and 26 had cerebral hemorrhage; 121 were male and 114 were female; mean age was 78.8 (11.6) years; and mean length of stay was 32.7 (18.1) days. No imputation was performed because the rate of missing values was less than 10% in all cases and missing variables were not used in this analysis [27]. Association of mRS on discharge with ability to turn over at the start of in-hospital rehabilitation, On univariate logistic regression analysis, ability to turn over was associated with mRS at discharge as the objective variable with an Odds Ratio (OR) of 8.6 (95% Confidence Interval (CI): 3.6-20.6, p<0.01).
On multivariate logistic regression analysis, mRS at discharge was the objective variable and the ability to turn over; gender, age, pre-hospitalization mRS, and paralysis status were the explanatory variables. OR for the ability to turnover was 5.6 (95% CI: 2.3-13.9, p<0.01) and the C-statistic was 0.82 (95% CI: 0.77-0.87). These results are listed in Table 2.
| Univariate analysis | Multivariate analysis* | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | AUC | 95% CI |
| 8.6 | 3.6-20.6 | <0.01 | 5.6 | 2.3-13.9 | <0.01 | 0.82 | 0.77-0.87 |
Note: OR: Odds Ratio; 95%CI: 95% Confidence Interval; AUC: Area Under the Curve
*Multivariate analysis was adjusted for age, gender, pre-hospitalization mRS, and presence of paralysis as confounders
Table 2: Association of mRS on discharge with ability to turn over at the start of in-hospital rehabilitation.
Sensitivity analysis
Sensitivity analysis to examine the relationship between mRS at discharge and the ability to turn over was performed by stratifying by age (<70 years, 70-84 years, and ≥ 85 years), gender (male and female), and pre-hospital mRS (mRS: 0,1,2 and mRS:3,4). On agestratified analysis, no patient aged 70 years or younger was unable to turn over, while the OR for patients aged 70 to 84 years was 6.7 (95% CI: 1.4-30.9, p=0.02) and the OR for patients aged 85 years or older was 4.2 (95% CI: 1.3-13.6, p=0.02). In terms of gender, the OR for males was 10.0 (95% CI: 1.3-79.0, p=0.03) while that for females was 4.6 (95% CI: 1.6-12.9, p<0.01). Regarding pre-hospital mRS, the OR for mRS: 0,1,2 was 8.5 (95% CI: 2.4-29.7, p<0.01) while that for mRS:3,4 was 3.1 (95% CI: 0.8-11.6, p=0.10). These results are listed in Figure 2.
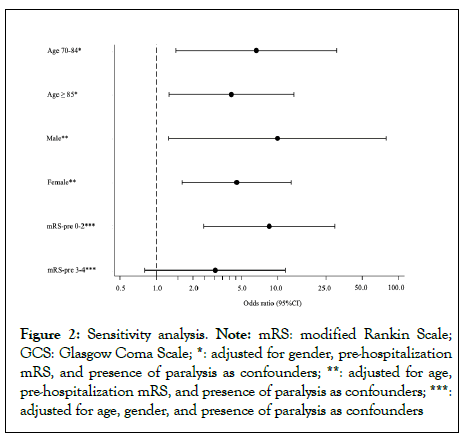
Figure 2: Sensitivity analysis. Note: mRS: modified Rankin Scale; GCS: Glasgow Coma Scale; *: adjusted for gender, pre-hospitalization mRS, and presence of paralysis as confounders; **: adjusted for age, pre-hospitalization mRS, and presence of paralysis as confounders; ***: adjusted for age, gender, and presence of paralysis as confounders
In this study, we found an independent relationship between the ability to turn over at the start of rehabilitation and bedridden status at discharge in patients hospitalized with acute cerebrovascular disease. Patients classified as “turning over impossible” had an OR of a bedridden state at discharge of 5.6 (95% Confidence Interval (CI) 2.3–13.9, p<0.001) and C-statistic of 0.82 (95% CI 0.77-0.87). Further, on sensitivity analysis, we found the results to be similarly significantly different by gender, age over 70 years, and pre-hospital mRS: 0-2. These findings support our hypothesis that the ability to turn over at the start of rehabilitation is a predictor of bedridden status at discharge. To our knowledge, this is the first study to show this association.
In the rehabilitation of patients with acute cerebrovascular disease, turning over is a basic trunk rotation movement that can be easily evaluated and subject to intervention from the early stage of the disease. Previous reports have shown that turning over reflects the impact of cerebrovascular disease on trunk and limb function [28,29]. Trunk function in turn has an effect on sitting and standing balance [10,30] and is more important than upper and lower extremity function in predicting the prognosis of physical function in stroke patients [31]. Moreover, trunk rotation function may predict the recovery of motor function and activities of daily living [32], suggesting the importance of trunk function as a predictor of physical function in patients with cerebrovascular disease. Our present finding that the ability to turn over at the initiation of rehabilitation may predict a bedridden state at discharge supports these previous studies.
Although our results show a strong relationship between turning over and future bedridden state, stratified analysis showed no significant relationship in patients aged under 70 years and prehospital mRS:3,4. We consider that this is because younger patients are said to have a relatively better prognosis than older patients [33], and because the decline in physical and cognitive function due to frailty becomes more pronounced with age [11]. This finding also reflects previous reports that younger patients with cerebrovascular disease have a better neurological, functional, and cognitive prognosis than older patients [9,34]. Pre-admission mRS: 3,4 is not consistent with a previous study [11], likely due to the small sample size in our study. Therefore, the adjustment factor may have caused over-fitting, resulting in inadequate analysis. Future studies need to enroll an increased number of subjects.
Strength of this study is the use of turning over to evaluate trunk function. This an easily measurable index which can be performed at the bedside in a few minutes and without tools. Further, it is relatively easy to implement in clinical settings and can be evaluated safely and quickly even under acute management, with little error among raters. In addition, several studies have verified its reliability in physical function assessment scales including turning over [35-37].
Several limitations of our study warrant mention. First, it was a single-center study, and its generalizability may therefore be low. However, our hospital is the only tertiary care institution in a regional area with a population of approximately 370,000, and we believe that our study represents the actual situation of stroke in the region. Second, because of the retrospective nature of the study,the presence of unmeasured confounders cannot be excluded. However, we systematically identified important confounders by literature review and adjusted for them as much as possible, hence we consider that their influence is likely small. Third, some patients who were bedridden at the time of transfer to other medical institutions subsequently recovered after transfer. However, the period of hospitalization in our institution was shorter for those who could turn over than for those who could not, suggesting that the recovery of ADL in these patients was shorter. Accordingly, we considered that this bias would likely dilute the association of turning over with the bedridden state, and therefore lead to underestimation of the association. Veerbeek, et al. reported that patients with poor sitting balance (Trunk Control Test–sitting; 30 seconds) and hemiplegic leg strength (Motricity Index leg; eg, visible contraction for all 3 items, or movement against resistance but weaker for 1 item) on day 9 post-NO stroke had a 10% gait gain at 6 months. This in turn suggests that the possibility that the patient will remain bedridden after discharge from the hospital cannot be ruled out [38]. Fourth, the sample size is relatively small. However, we believe that the power was sufficient because it met the sample size estimated in the research planning stage. Since the sample size was small for stratified analysis, however, future studies should include an increased number of subjects, and compare findings by length of hospital stay, disease type etc. Fifth, we did not use an evaluation scale that includes turning over. Rather, turning over was extracted from the comprehensive rehabilitation implementation plan, which also meant that we were not able to evaluate turning over on the paralyzed and non-paralyzed sides separately.
To improve predictive ability, future studies should use a rating scale that differentiates turning over on the paralyzed and non-paralyzed side. We found that the ability to turn over at the beginning of rehabilitation is a predictor of the bedridden state at the time of hospital discharge. Turning over can be easily measured and implemented in clinical settings, warranting further investigation to determine how measuring turning over affects the clinical environment of stroke rehabilitation.
We would like to express our deepest gratitude of the Rehabilitation Department, Department of Neurology, Department of Neurosurgery, for their cooperation in conducting this study. We also thank Guy Harris, of DMC Corp. (www.dmed.co.jp) for editing drafts of this manuscript.
This research was conducted with the Aso Iizuka Hospital Clinical Research Grant.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Nishida H, Sasaki S, Terashita S, Yokote T, Imoto T, Yamashita T (2022) Association between the Ability to Turn Over on Admission and Bedridden State at Discharge for Stroke. Int J Phys Med Rehabil. S21:002.
Received: 01-Aug-2022, Manuscript No. JPMR-22-18614; Editor assigned: 05-Aug-2022, Pre QC No. JPMR-22-18614 (PQ); Reviewed: 25-Aug-2022, QC No. JPMR-22-18614; Revised: 01-Sep-2022, Manuscript No. JPMR-22-18614 (R); Published: 08-Sep-2022 , DOI: 10.35248/2329-9096.22.10.646
Copyright: © 2022 Nishida H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.