Chemotherapy: Open Access
Open Access
ISSN: 2167-7700
ISSN: 2167-7700
Research Article - (2024)Volume 12, Issue 4
Introduction: Kidney cancer is an immunogenic tumor and inflammation plays a role in tumor formation and progression. The aim of this study was to investigate the effect of biochemical markers in predicting treatment response in patients with metastatic Renal Cell Carcinoma (RCC) receiving nivolumab treatment.
Methods: Patients aged ≥ 18 years who received nivolumab treatment for metastatic RCC between February, 2022 and January, 2024 were evaluated. Cellular blood count was evaluated at the diagnosis of metastatic disease and periodically during nivolumab treatment. We measured the cell count in peripheral blood at admission and calculated the inflammation indices obtained from the blood cell count as follows: MCVL (Mean Corpuscular Volume/Lymphocytes) and IIC ((mean corpuscular volume *width of erythrocyte distribution* neutrophils/(lymphocytes*1000)).
Results: The study included 99 patients. The mean age of the patients was 58.32 ± 11.95 (19-87). 75.8% of the patients had clear cell and 8.1% had papillary type pathology. When the disease control status was evaluated at 3 months after nivolumab treatment, it was seen that 48 patients had partial response and 18 patients remained stable. The 3rd month Disease Control Rate (DCR) was 68.7%. When the ICIC and MCVL rates of the patients were examined, it was determined that the ICC and MCVL values of the patients who showed progression were higher (p=0.038 for ICC, p=0.040 for MCVL). The ICC cut-off value was determined as 15.50. With this cut-off value, disease control can be predicted with 77% sensitivity and 64% specificity (AUC: 0.743 CI 95% (0.647-0.838); p<0.001). MCVL cut-off value was determined as 65.15. This cut-off value can predict disease control with 65% sensitivity and 61% specificity (AUC: 0.674, CI: 95% (0.564-0.783), p=0.006).
Conclusion: Cumulative Inflammatory Index (ICC) and MCVL have a significant effect on disease-free survival and overall survival in predicting success of nivolumab treatment for metastatic renal cell carcinoma.
Nivolumab; Inflammatory marker; Acute pancreatitis; Neutrophils
Renal cell carcinoma accounts for 2%-3% of all cancers [1]. At the time of diagnosis, 45% of patients have localized disease, 25% have locally advanced disease and 20%-30% have metastatic disease. One in three patients treated for localized disease may develop metastatic disease in the future [2]. There are many factors affecting the prognosis of patients with metastatic Renal Cell Carcinoma (mRCC), such as histopathology, TNM, stage and clinical factors [3,4].
Kidney cancer is characterized by high production of proangiogenic factors, including Vascular Endothelial Growth Factor (VEGF) and platelet-derived growth factor. Therapeutic approaches to inhibit the VEGF pathway have led to the introduction of Tyrosine Kinase Inhibitors (TKI) such as sunitinib and pazopanib into clinical practice [5,6]. Today, the new generation PD-1 antibody nivolumab is used in the treatment of metastatic RCC and positive results are obtained [7].
Kidney cancer is an immunogenic tumor where inflammation plays a significant role in tumor formation and progression [8]. Neutrophil-To-Lymphocyte Ratio (NLR), Platelet-To-Lymphocyte Ratio (PLR) and Lymphocyte-To-Monocyte Ratio (LMR) are indirect biomarkers of systemic inflammation and have been widely investigated in oncology patients treated with chemotherapy and TKIs [9,10].
In the COVID-19 pandemic that has recently affected the world, new markers used to predict acute pancreatitis have come to the fore. These are the MCVL and the Cumulative Inflammatory Index (IIC). Both biomarkers have been used safely.
The aim of this study was to investigate the effect of biochemical markers in predicting treatment response in metastatic RCC patients receiving nivolumab treatment.
This study included patients over the age of 18 who received nivolumab treatment for metastatic RCC between February, 2022 and January, 2024. The study was approved by the ethics committee (2024/010.99/6/9). The study was designed in accordance with the Declaration of Helsinki and written informed consent was obtained from each patient.
Cellular blood counts were assessed at diagnosis of metastatic disease and periodically during nivolumab treatment. Age, gender, body mass index, histology, International Association of Urological Pathologists/World Health Organization grading, previous nephrectomy and number of metastatic sites were collected and analyzed.
The results of the first blood test within the first 24 h after admission were also taken into account: White Blood Cell Count (WBC), Neutrophil Count (NEU), Lymphocyte Count (LYM), Monocyte Count (MON), Platelet Count (PLT), Red Cell Distribution Width (RDW) and Mean Corpuscular Volume (MCV). We collected the data obtained from the blood by obtaining the laboratory tests available after the first blood draw. We measured the cell count in peripheral blood at the time of admission and calculated the inflammation indices obtained from the blood cell count as follows: MCVL (Mean Corpuscular Volume/Lymphocytes) and IIC ((mean corpuscular volume * width of erythrocyte distribution* neutrophils/(lymphocytes*1000)).
Statistical analysis
The analysis was performed using the SPSS 25.0 software (IBM, NY, USA). The distribution of data was evaluated using the Kolmogorov-Smirnov test. Comparison of patients with and without progression was performed using an independent sample t-test. Receiver Operating Characteristics (ROC) curve analysis was performed to determine values indicating progression. Survival was evaluated using Kaplan-Meier curve regression analysis. Statistical significance was determined as p<0.05.
Among these patients, the mean age of the patients was 58.32 ± 11.95 (19-87). 75.8% of the patients had clear cell and 8.1% had papillary type pathology. When metastasis sites were evaluated, multiple organ metastasis was detected in 58 patients, 17 patients had lung, 17 patients had bone and 4 patients had brain metastasis. Demographic data and laboratory parameters of the patients before nivolumab treatment are given in Table 1. The table shows the demographic and clinical characteristics of patients with metastatic RCC undergoing nivolumab treatment.
| Parameter | (%) | Mean ± Standard Deviation (Min-Max) |
|---|---|---|
| Age | - | 58.32 ± 11.95 (19-87) |
| Subtype | ||
| Clear cell | 75 (75.8%) | - |
| Papillary | 8 (8.1%) | - |
| Chromophobe | 1 (1%) | - |
| Sarcomatoid | 2 (2%) | - |
| Other | 13 (13.1%) | - |
| Metastasis site | ||
| Lung | 17 (17.2%) | - |
| Bone | 17 (17.2%) | - |
| Lymph node | 2 (2%) | - |
| Liver | 1%-1% | - |
| Brain | 4 (4%) | - |
| Multipl | 58 (58.6%) | - |
| White Blood Cells (WBC) | - | 6.63 ± 2.96 (1.03-14.60) |
| Red Blood Cells RBC | - | 3.92 ± 0.68 (2.75-5.81) |
| Hemoglobin HGB | - | 11.28 ± 2 .02 (3.90-16.90) |
| Hematocrit (HCT) | - | 35.39 ± 5.23 (25.20-48.90) |
| Mean Corpuscular Volume (MCV) | - | 90.86 ± 9.14 (69.50-111.50) |
| Platelet (PLT) | - | 275.50 ± 111.12 (104-565) |
| Neutrophil | - | 4.65 ± 2.53 (0.90-13.18) |
| Lymphocyte | - | 1.65 ± 0.84 (0.39-4.40) |
| RDW (Red Cell Distribution Width) | - | 55.64 ± 21.36 (15.20-89.20) |
| Cumulative Inflammatory Index (ICC) | - | 17.34 ± 15.49 (1.20-94.90) |
| MCVL | - | 69.26 ± 36.77 (20.88-226.92) |
Table 1: Patient demographics and clinical characteristics.
When the disease control status was evaluated at 3 months after nivolumab treatment, it was seen that 48 patients had partial response and 18 patients remained stable. The 3rd month DCR was 68.7% (68/99).
After treatment, 6th month DCR was 51.5% (51/99) and 12th month DCR rate was 25.3% (25/99). Median progression-free survival was 6 months (2-36) and overall survival was 12 months (2- 45) (Table 2). The table shows the disease control status at different time points (3rd month, 6th month and 12th month) in patients with metastatic RCC undergoing nivolumab treatment.
| Timepoint | Progressive Disease (PD) | Stable Disease (SD) | Partial Response (PR) | Complete Response (CR) | Disease Control Rate (DCR) |
|---|---|---|---|---|---|
| 3rd month | 19 (19.2%) | 18 (18.2%) | 48 (48.5%) | N/A | 68/99 (68.7%) |
| 6th month | 14 (14.1%) | 9 (9.1%) | 38 (38.4%) | 1 (1%) | 51/99 (51.5%) |
| 12th month | 13 (13.1%) | 5 (5.1%) | 19 (19.2%) | 1 (1%) | 25/99 (25.3%) |
Table 2: Disease control rates.
After nivolumab treatment, the 3rd month disease control patient rate was determined as 68.7%. When the ICIC and MCVL rates of the patients were examined, the ICC and MCVL values of the patient’s showing progression were determined to be higher (p=0.038 for ICC, p=0.040 for MCVL) (Table 3). The table compares laboratory parameters between patients with metastatic RCC who have achieved Disease Control (DC) and those who have not by the 3rd month of treatment.
| DC (-) (31 patients) | DC (+) (68 patients) | ||||
|---|---|---|---|---|---|
| Disease control response | Mean | Mean | p-values | ||
| 3rd month | |||||
| WBC | 6.27 | 2.34 | 6.80 | 3.20 | 0.405 |
| RBC | 3.65 | 0.66 | 4.04 | 0.65 | 0.211 |
| HGB | 10.37 | 2.21 | 11.69 | 1.80 | 0.117 |
| HCT | 33.41 | 4.32 | 36.29 | 5.39 | 0.056 |
| MCV | 91.02 | 9.05 | 90.79 | 9.24 | 0.908 |
| PLT | 244.35 | 108.28 | 289.70 | 110.26 | 0.059 |
| Neutrophil | 4.34 | 2.10 | 4.79 | 2.71 | 0.412 |
| Lymphocyte | 1.31 | 0.51 | 1.80 | 0.91 | 0.007 |
| RDWT | 58.61 | 20.58 | 54.28 | 21.72 | 0.352 |
| ICC | 23.69 | 11.44 | 16.78 | 16.62 | 0.038 |
| MCVL | 79.38 | 32.15 | 64.64 | 38.02 | 0.04 |
Table 3: Comparison of laboratory parameters of patients according to their 3rd month disease control status.
The number of patients whose disease was controlled at 12 months after nivolumab treatment was 25 (25.3%). When the inflammatory markers of patients with and without progression were compared, it was found that ICC and MCVL values were higher in patients with progression (p=0.029 for ICC, p=0.009 for MCVL) (Table 4). The table compares laboratory parameters between patients with metastatic RCC who have achieved Disease Control (DC) and those who have not by the 12th month of treatment.
| DC (-) (74 patients) | DC (+) (25 patients) | ||||
|---|---|---|---|---|---|
| Disease control response | Mean | Standard Deviation (SD) | Mean | Standard Deviation (SD) | p-values |
| 12th month | |||||
| WBC | 6.73 | 2.83 | 6.34 | 3.35 | 0.571 |
| RBC | 3.83 | 0.68 | 4.18 | 0.59 | 0.023 |
| HGB | 10.97 | 2.10 | 12.18 | 1.47 | 0.09 |
| HCT | 34.62 | 5.20 | 37.65 | 4.73 | 0.012 |
| MCV | 90.74 | 9.02 | 91.19 | 9.65 | 0.833 |
| PLT | 277.87 | 122.78 | 268.48 | 67.06 | 0.717 |
| Neutrophil | 4.59 | 2.71 | 4.83 | 1.93 | 0.684 |
| Lymphocyte | 1.47 | 0.59 | 2.19 | 1.19 | 0.001 |
| RDWT | 57.09 | 20.74 | 51.35 | 22.99 | 0.248 |
| ICC | 20.60 | 16.41 | 14.05 | 11.15 | 0.029 |
| MCVL | 74.15 | 38.16 | 54.78 | 28.29 | 0.009 |
Table 4: Comparison of laboratory parameters of patients according to their 12th month disease control status.
The ICC cut-off value, which evaluates whether the disease is under control at 3 months after treatment, was determined as 15.50. With this cut-off value, disease control can be predicted with 77% sensitivity and 64% specificity (AUC: 0.743 CI: 95% (0.647-0.838); p<0.001) (Figure 1).
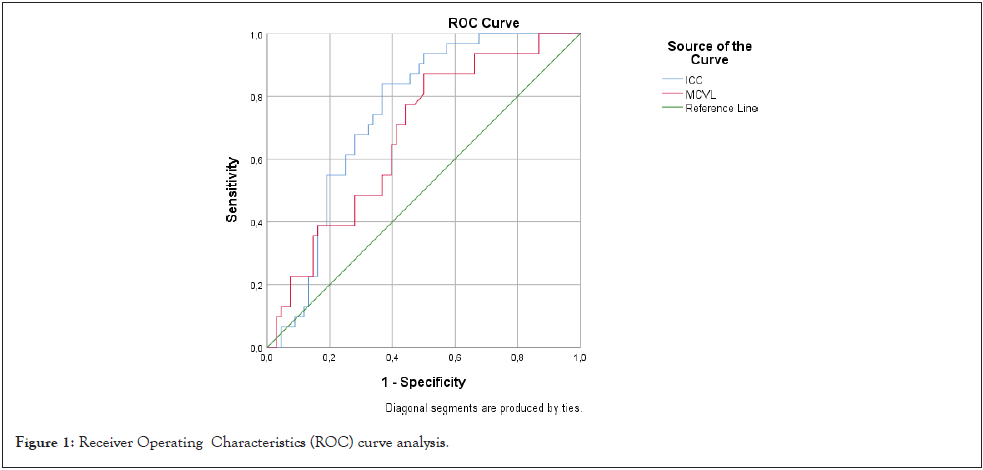
Figure 1: Receiver Operating Characteristics (ROC) curve analysis.
The MCVL cut-off value for whether the disease is under control at 3 months after treatment was determined as 65.15. This cutoff value can predict disease control with 65% sensitivity and 61% specificity (AUC: 0.674, CI: 95% (0.564-0.783), p=0.006) (Figure 1).
When PFS was evaluated according to ICC>15.50 or <15.50, it was found that patients with >15.50 had shorter PFS (13.8 months vs. 38.6 months, HR: 6.78 CI 95%(2.7-37.04), p=0.009) (Figure 2).
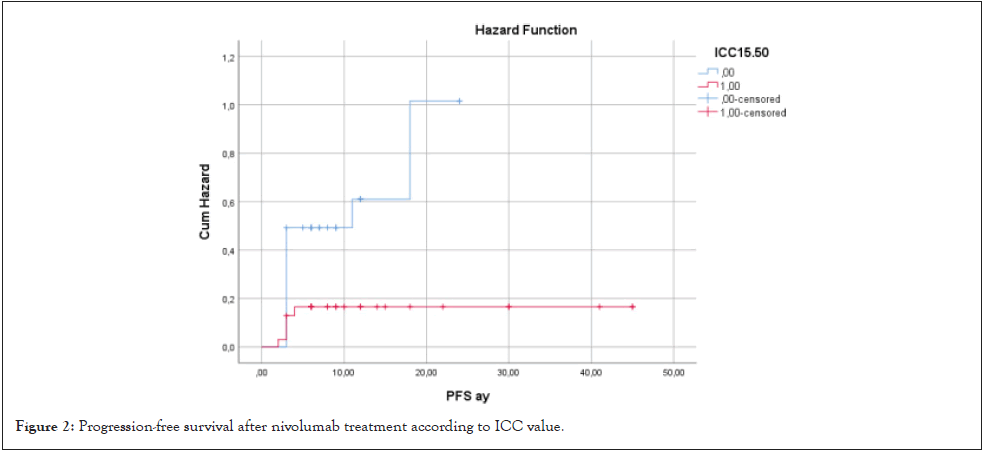
Figure 2: Progression-free survival after nivolumab treatment according to ICC value.
When PFS was compared according to MCVL cutoff value 65.15, PFS of the group with MCVL>65.15 was shorter, but it was not statistically significant (18.2 months vs. 28.3 months HR: 3.18, p=0.074) (Figure 3).
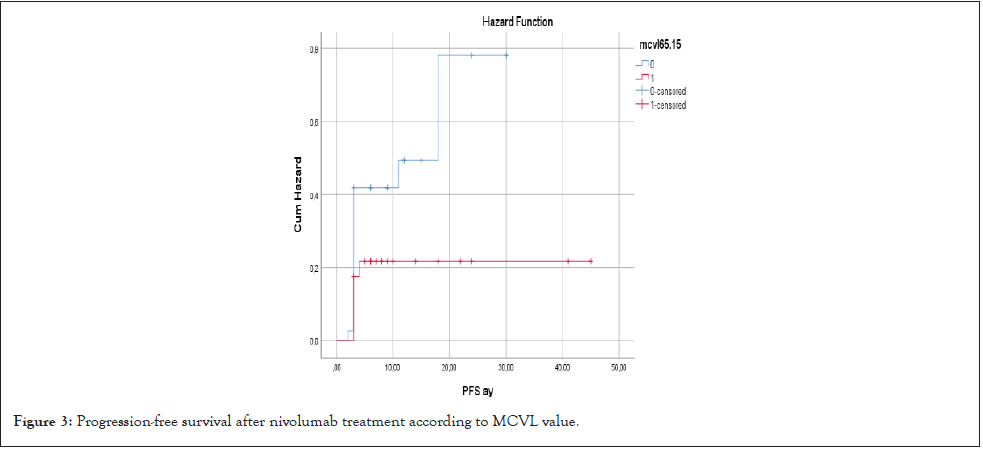
Figure 3: Progression-free survival after nivolumab treatment according to MCVL value.
When OS was compared according to ICC cutoff value of 15.50, OS was found to be shorter in the group with ICC >15.50 (19.01 months vs. 30. 35 months; HR: 3.98 CI 95% (1.02-6.68) p=0.048) (Figure 4).
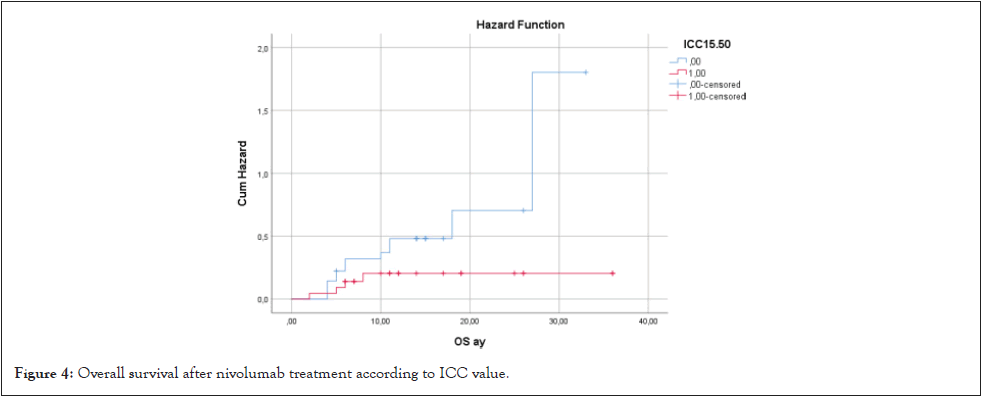
Figure 4: Overall survival after nivolumab treatment according to ICC value.
When OS was compared according to MCVL cutoff value of 65.15, OS was found to be shorter in patients with MCVL>65.15, but no statistical significance was found (21.6 months vs. 24 months; HR: 2.07 (0.5-3.1), p=0.108) (Figure 5).
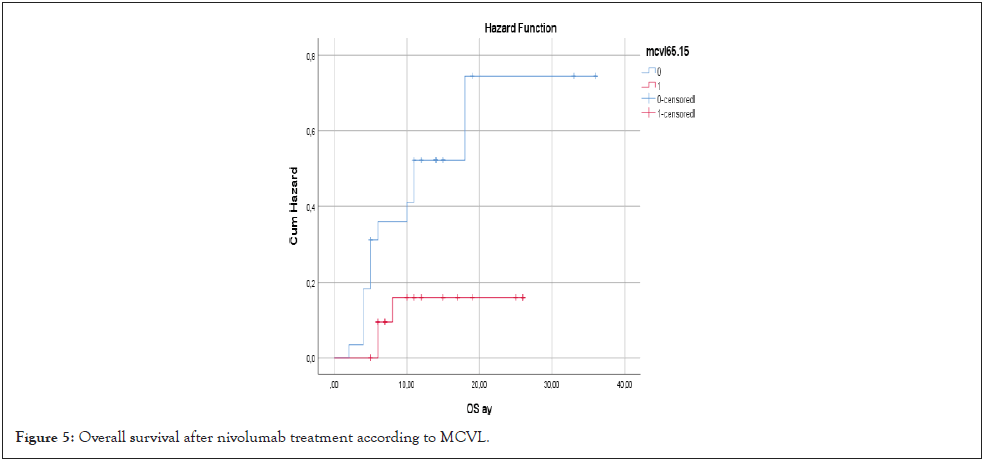
Figure 5: Overall survival after nivolumab treatment according to MCVL.
At the end of the study, it was observed that ICC and MCVL had a predictive effect on progression in patients receiving nivolumab treatment for metastatic renal cell carcinoma. In addition, significant differences were found in PFS and OS times according to ICC and MCVL cut-off values (Supplementary Table 1). Inflammation promotes tumor formation and is considered a key mechanism in the maintenance and spread of tumors after their formation [11]. It has also been observed that increased systemic inflammation affects the immune response, causing tumor cells to escape immune surveillance [12]. Neutrophils, one of the inflammatory markers measured in peripheral blood, mediate the production of cancer-associated chemokines and cytokines. They also play a role in the proliferation, invasion and metastatic spread of tumor cells and also induce drug resistance [13].
The increase in neutrophils and platelets has been found to be associated with the progression of tumor cells. The decrease in the number of lymphocytes may be responsible for the weak and inadequate immune response against tumors and has also been associated with poor prognosis, as have the increase in neutrophils and platelets [14]. Systemic inflammatory markers such as ICC are important in this regard. In our study, it was determined that ICC and MCVL values were different in patients who responded and did not respond to nivolumab treatment due to the decrease in lymphocytes from systemic markers and these markers were higher in the group showing progression. This may be effective in predicting the progression of the disease and the response to treatment.
Nowadays, inflammatory markers have an important place in disease diagnosis and follow-up. Many biomarkers are used in the literature, especially in the prognosis of cancer disease [13-15]. Systemic inflammatory index score has been found to be associated with prognosis in metastatic patients using immune checkpoint inhibitors. Another study has shown that inflammatory biomarkers are associated with predicting the success of immunotherapy used in the treatment of metastatic urothelial cell carcinoma [16]. However, the most important shortcoming of each biomarker is that these markers can be affected very easily and give false results. For this reason, markers that are a combination of many factors can give more controlled results. In a study examining the short-term effect of anti-Programmed Death-1 (PD-1) treatment used for esophageal squamous cell carcinoma, it was determined that inflammatory markers can reflect the results in the short term [17]. In patients with advanced pancreatic cancer, inflammatory biomarkers have been shown to have effects on progression and overall survival in PD-1 treatment [18].
ICC and MCVL, which were first used to determine the prognosis in acute pancreatitis attacks in COVID-19 patients, contained significant results [19]. While MCVL was found to be associated with the occurrence of complications, ICC was found to have an effect on mortality. Inspired by this study, when the ICC and MCVL values of the patients were compared in order to determine the response to Nivolumab treatment at an early stage, it was seen that both MCVL and ICC had significant effects on PFS and OS. It was observed that the ICC Cutoff value was 15.50 and patients with an ICC value above this value had shorter PFS and OS periods. Similarly, an MCVL value above 65.15 also has negative effects on PFS and OS. In this study, determining the ICC and MCVL values before treatment in order to predict the response to nivolumab treatment applied in the treatment of RCC emerges as a parameter that can significantly determine the response to treatment and prognosis.
The study has some limitations. The first of these is the small number of patients. Another limitation is the short follow-up period. The lack of comparison with other serum biomarkers is among the other limitations. However, the analyses show that ICC and MCVL predict prognosis with high sensitivity and specificity.
In conclusion, both ICC and MCVL, emerging as new inflammatory markers, play a critical role in predicting the disease-free survival and overall survival outcomes in patients undergoing nivolumab treatment for metastatic renal cell carcinoma. Their impact on the treatment success highlights the potential of inflammation-related biomarkers in therapeutic response. However, further studies with larger patient cohorts are essential to better understand the complex relationship between inflammation and tumor behavior and to refine these markers for the clinical use in assessing treatment efficacy and improving the patient outcomes in this context.
This study was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. It was approved by the Ethics Committee of the Women and Children's Hospital of Hunan, Changsha, China (Approval Number: 2024-13).
Since this study is retrospective, no consent form is required.
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
All authors contributed equally to this article.
The authors declare that there are no conflicts of interest regarding the authorship and publication of this article.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Surmeli H, Isik D, Bal H, Tunbekici S, Kinikoglu O, Altintas YE, et al. (2024). Association of New Inflammatory Markers Cumulative Inflammatory Index (ICC) and Mean Corpuscular Volume/Lymphocyte (MCVL) with Anti PD-1 Antibody Nivolumab Treatment Response in Metastatic Renal Cell Carcinoma. Chemo Open Access. 12:227.
Received: 18-Nov-2024, Manuscript No. CMT-24-35549; Editor assigned: 20-Nov-2024, Pre QC No. CMT-24-35549 (PQ); Reviewed: 04-Dec-2024, QC No. CMT-24-35549; Revised: 11-Dec-2024, Manuscript No. CMT-24-35549 (R); Published: 19-Dec-2024 , DOI: 10.35248/2167-7700.24.12.227
Copyright: © 2024 Surmeli H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited