
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2020)Volume 10, Issue 6
Olanzapine, a widely used antipsychotic, causes significant iatrogenic side effects of obese leading to non-compliance of antipsychotics in schizophrenia patients. However, the mechanism of olanzapine creating obese of metabolic disorders has remained elusive till now. Adipocyte proliferation was believed as general reasons for obesity-related diseases, convinced from our olanzapine-induced obese rat model. Our results demonstrated adipocyte proliferation through significantly elevating P-PI3K and P-AKT cascades in chronic treatment of olanzapine. Interestingly, significant activations of apoptosis through Klotho-Egr-1-MnSOD pathway were also observed in the obese rat model. We hypothesized that the activation of the apoptotic pathway could elicit a feedback response in adipocyte proliferation after olanzapine chronic treatments. Our results demonstrated that olanzapine-induced apoptosis responses on 3T3-L1 adipocytes in a dose-dependent manner, while significant adipocyte proliferation was only visible in a range of 5 μM olanzapine which is a general dose concentration in clinic applications. Furthermore, administrations of recombinant Klotho peptide could restrict the activation of apoptosis by olanzapine in clinic dosage and then inhibited feedback response of adipocyte proliferation. The current study shed lights on appeasing olanzapine-induced obese syndromes through a new target of blocking apoptotic activation by recombinant klotho and then improving the therapeutic effect of olanzapine.
Olanzapine; Obesity; Apoptosis; Klotho; MnSOD; Egr-1
Olanzapine is a second-generation atypical antipsychotic (AAP), which has distinct advantages in controlling patients with psychotic symptoms and reducing readmission rate. It has become the first- line drug for the treatment of schizophrenia and other related mental illnesses [1]. In the meantime, olanzapine is prone to cause metabolic syndrome, including significant weight gain, and to a certain extent affecting changes in blood glucose, blood lipids and insulin levels, which severely affect the medication compliance and treatment outcomes of patients with schizophrenia [2,3]. Studies have shown that olanzapine-induced abnormal weight gain is a significant factor in the development of metabolic syndrome [4]. However, the mechanism by which olanzapine induces abnormal obese has not yet been explored.
Numerous studies have shown that apoptotic cascade activations were strictly related to obesity. Naim et al. showed that abnormal weight gain and insulin resistance was associated with increased apoptosis in adipocytes [5]. Similar findings were also observed in several different groups. Feng et al. demonstrated that increasing apoptotic levels in white-fat cells of mouse models could lead to metabolic disorders and obesity as well [6]. Liang et al. strongly argued significant correlations between high fat-induced obesity and adipocyte apoptosis [7]. Obsess has long been ascribed to inflammation activity in fat tissue triggering triglyceride overload to adipocytes and ultimately leading to apoptosis turn-over in fat cells. Increased apoptosis was further postulated in turn to fire intensive macrophage infiltration in adipose tissue to aggravate obesity further [8]. Our previous work also determined significant inflammatory activation and obese both in olanzapine-administered mouse and rat insulin-resistance models [9]. We thenceforth hypothesized that olanzapine-induced obesity was caused by a feedback response chain of inflammation-apoptosis activation pathway.
Along with this postulation, our proteome data witnessed the significant abnormal variations in inflammation-related gene Egr-1, and aging-related gene Klotho after chronic olanzapine administration in rodent models. More importantly, current study further confirmed significant increases in p-AKT and p-PI3K levels of white adipose tissue after chronic olanzapine administration in a rat model, suggesting substantial cell proliferation owing to activation in the well-known PI3K-AKT signaling pathway.
The Klotho gene first discovered in 1997 encoding two isoforms, klothoα (KLA) and klothoβ (KLB). KLA, a single transmembrane protein of 1,014 amino acids in length, expressed primarily in the kidney, parathyroid, and choroid plexus, and was found mainly roles in aging-suppression [10]. KLB a single transmembrane protein of 1,043 amino acids, sharing 41.2% homology with KLA, was found mainly expressed in liver, adipose tissue, and pancreas [11]. Interestingly, Lin et al. demonstrated that the decreased KLB in pancreatic β cells caused upregulations in apoptosis, oxidative damage, and DNAJC3 levels (indicators for endoplasmic reticulum stress levels). Specifically, overexpression of klotho could prevent apoptosis of pancreatic beta cells and prevents Streptozotocin (STZ)-induced diabetes [12,13]. On the other hand, Egr-1, an early growth response factor, mediates insulin sensitivity and promotes the release of adipose tissue inflammatory cytokines [14]. It has been reported that Klotho can down-regulate Egr-1 by inhibiting TGF-β1/ Smad3 signaling in DKD (diabetic nephropathy), and silenced Klotho gene would up-regulate Egr-1 vice versa [15]. Manganese superoxide dismutase (MnSOD) is a mitochondrial antioxidant enzyme (an ER stress factor) involving in the conversion of oxygen free radicals to hydrogen peroxide [16]. MnSOD downregulation caused an increase in reactive oxygen species (ROSs) and apoptosis of the cells [17,18] via p53-induced apoptotic pathway [19,20].
Our current study demonstrated that decreased Klotho activity with overexpression of Egr-1 and decreased MnSOD levels in chronic olanzapine-induced insulin-resistance rodent models [21]. In 3T3- L1 adipocyte study, we further testified that overexpression of Egr- 1 and decreased MnSOD were along with olanzapine treatment and the apoptosis activation with Klotho downregulation in 3T3-L1 was confirmed in a dose-dependent manner. Most of all, the 3T3-L1 adipocyte increment significant only in line with the activation of the PI3K-AKT pathway and apoptotic activation in 5 μM olanzapine treatment which is the general dosage range for clinic administration [22]. The overdose of olanzapine, more than 10 μM could only elicit apoptosis significantly without adipocyte growing increment. Furthermore, application of recombinant klotho peptide could attenuate apoptotic reaction and block growing increase induced by 5 μM olanzapine treatment to 3T3- L1adipocyte.
Current study provided a new mechanism which obesity caused by chronic olanzapine treatment in the clinic was probably associated with adipocyte over-proliferation response due to inflammation- apoptosis activation involving in the downregulation of klotho and MnSOD but over-activated inflammation factor Egr-1. The association of olanzapine-induced metabolic disorders in abnormal obese and activated apoptotic pathway could provide a new view for reducing drug side effects and improving drug compliance in antipsychotic treatments in the future.
Antibodies and reagents
Anti-Klotho (ab203576), Anti-P-PI3K (ab182651), Anti-MnSOD (ab13533), Anti-GAPDH (ab37168) were purchased from Abcam (Cambridge, UK). Anti-Akt(#9272), Anti-P-Akt(#4060), Anti- PI3K(#4292) were purchased from Cell Signaling Technology (Boston, MA, Massachusetts, America). Secondary Antibody: HRP-Goat Anti-Rabbit IgG (H+L) (AS014) was purchased from Abclonal (Wuhan, China). Olanzapine (LRAA9505, 98% HPLC grade) was purchased from Sigma (St. Luis, MO, USA), Annexin V-FITC Apoptosis Detection Kit was respectively purchased from BD Biosciences (556547) and Beyotime (C1062L).
Model development for Olanzapine induced obese
210-230 g SD rat (Purchased from Hubei Provincial Center for Disease Control) were housed with a 12-h light/dark cycle (22-24, 55 ± 10% humidity), water and food ad libitum. After acclimation for 1 week, weight-matched rats were divided randomly into Saline-treatment (control) and olanzapine-treatment (model) groups (n=15/group). Rats in the model group were intragastrically administered with olanzapine (1.5 mg/10 ml/kg) once every day during 8-week treatment one hour before each nightfall, and the control group was given an equal volume of physiological saline. The changes of rat body weights were measured weekly, but blood glucose and insulin levels (Elorite Item, E-EL-R2466c) were measured biweekly starting from 2nd week of olanzapine or saline treatment. At the end of 8-week treatment, rats were then anesthetized, blood samples and tissue specimens were collected for later analyses.
Oral glucose tolerance tests
The oral glucose tolerance test (OGTT) was performed after the end of the 8-week administration of olanzapine, and the area under the blood glucose curve was calculated. All rats were administered with 20% D-glucose (2 g/kg) before start taking Rat tail blood samples. Bloods (500 μl) were collected at 0, 15, 30, 60, 90 and 120 min to perform OGTT assays from all rats of two groups by using commercial kits (Roche, Basel, Switzerland).
Mercury content was quantified using a calibration curve with known concentrations of mercury in the same dilute acid diluent as the samples. ICP-MS analysis of these standards and samples was performed by directly monitoring several mercury isotopes at 199, 200, 201, and 202 m/z. In addition to multiple isotope monitoring, the use of a helium collision cell minimized and/or eliminated potential polyatomic interferences. An on-line addition internal standard solution comprised of bismuth (209 m/z) was utilized to correct for any potential instrumental response variations observed during the analysis. The use of ICP-MS is critical for the analysis due to the higher selectivity and lower detection limits versus those of an ICP-OES type system. Final results are expressed as parts per million (PPM) of mercury.
Olanzapine and recombinant klotho treatments
Cell line 3T3-L1 used in this work was purchased from Shanghai Meixuan Biotechnology Co., Ltd. (Shanghai, China). Cells were cultured in DMEM medium (which contained 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin) in a 5% CO2, 37°C incubator. When cultures became 75% confluence, the cells were treated with Olanzapine (0 μM; 1 μM; 2 μM; 5 μM; 10 μM; 20 μM), with or without recombinant human Klotho (rKlotho; 1 μg/ml, R&D System) for 48 h.
Western blot analysis
Treated 3T3-L1 cells were homogenized and centrifuged to extract proteins. Protein concentrations were determined by BCA Protein Assay Kit (P0011, Beyotime Institute of Biotechnology, Nanjing, China). Cell lysates (30 μg each) were separated with 5-15% SDS- PAGE gel and transferred onto a nitrocellulose membrane. The blots were blocked with 5% non-fat milk PBS-Toween20, and then incubated overnight separately at 4 with the following antibodies, rabbit anti-Klotho (1:1000), rabbit anti-P-PI3K(1:1000), rabbit anti- MnSOD(1:2500), rabbit anti-GAPDH(1:5000), rabbit anti-Akt (1:1000), rabbit anti-P-Akt (1:1000), or rabbit anti-PI3K(1:1000). After removing the 1st antibody with complete wash, the blots were incubated with HRP-Goat Anti-Rabbit IgG (H+L)(1:5000), and the probed immunoreactive signals were visualized by Tanon chemiluminescence Bio ECL Plus (Tanon 5200, China).
Immunofluorescence
After treatment with olanzapine (5 μM) and Saline(Control) for 48 h, it was washed 3 times with PBS. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Anti-KL antibody was used at a dilution of 1:200. Alexa Fluor® 488-conjugated goat anti-rabbit IgG (1/500) (ab150077) was used as the secondary antibody. DAPI (blue) was used as the nuclear counterstain. This experiment was used to visually observe changes in the expression level of klotho protein after olanzapine administration.
ELISA assay for insulin
Take 200 mg of adipose tissue from the control group and olanzapine group, add the cold PBS to the glass homogenizer on the ice, and then centrifuge the homogenate 5000 × g for 5-10 minutes, take the supernatant. Commercially, the insulin content in the medium was determined using an ELISA insulin kit (Elabscience, E-EL-R2466c). This experiment was used to compare the level of insulin in white adipose tissue of olanzapine-administered rats and the control group.
Quantitative Real-Time PCR
Total RNA was isolated using RNA-spin Total RNA Extraction Kit (TIANGEN, Beijing, China). Real-time PCR was performed in a LightCycler 480 system (Roche, Rotkreuz, Switzerland) using the SYBR® Premix Ex TaqTM kit (RR047A, Takara, Japan). The primers for Klotho (Rat) were
5 ' - C TGGAGGTGCAGGAGATGACT-3' and 5 ' - AAAGGTTGATGCCGTCCAAC-3'
The primers for Caspase 3 (Rat) were
5 ' -GTATGCTTACTCTACCGCACCC-3' and 5'- C AGGGAGAAGGACTCAAATTCC-3'
The primers for GAPDH (Rat) were
5 ' - CGCTAACATCAAATGGGGTG-3' and 5 ' - CAGGGAGAAGGACTCAAATTCC -3'
The primers for Klotho (Mouse) were
5 '-GGCTTTCCTCCTTTACCTGAAAA-3' and 5 ' - CACATCCCACAGATAGACATTCG-3'
The primers for Caspase 3 (Mouse) were
5 ' - G G AGAAATTCAAAGGACGGG-3' and 5 ' - G C ATGGACACAATACACGGG-3'
The primers for GAPDH (Mouse) were
5 ' - TGAAGGGTGGAGCCAAAAG-3' and 5 ' - AGTCTTCTGGGTGGCAGTGAT-3'
The calculation formula was as follows:
ΔCt=Ct value of the target gene-Ct value of the reference gene
ΔΔCt=ΔCt of control group-ΔCt of the experimental group
The relative expression value of the target gene in the sample of the experimental group is 2ΔΔCt.
Cell viability assay
Cell viability of 3T3-L1 cells treated by Olanzapine (Sigma, USA) was determined by CCK-8 reagent (DOJINDO, Japan). The cells (1 × 10^4/ml) in the exponential growth phase, were seeded into 96-well plates plus 100 μl media per well. After 24 h of culture, the media was replaced by the fresh media with different concentrations of Olazapine (0,1,2,5,10, or 20 μM respectively) in quadruplicate wells with/without rKlotho peptides (1 ug/ml). After 48 hours of incubation, 10 μl CCK-8 solution was added to the cells and then incubated at 37 °C for 2 h. The absorbance of each well was then measured using a standard enzyme-linked immunosorbent assay at 450 nm. Cell viability percentage=(experimental group absorbance value/control group absorbance value)*100%.
Assessment of apoptotic adipocytes with flow cytometry
After treatment with Olanzapine or rKlotho, trypsinized cells were incubated with 5 μl of FITC Annexin V and 5 μl Propidium Iodide(PI)in 1 × binding buffer (BD Biosciences) for 15 min at room temperature according to the manufacturer’s manual. The immunofluorescence counts of stained cells were measured and analyzed by flow cytometry (BD FACS Calibur Instrument). Values are expressed as the percentage of fluorescent cells relative to the total cell count.
Assessment apoptotic adipocytes with confocal microscopy
Adipocyte 3T3-L1 cells were seeded on poly D lysine-coated chamber slides and then treated with 1, 2, 5, 10, 20 μM olanzapine or saline control. The cells were washed 2-3 times with PBS and then stained with 5 μl Annexin V-FITC and 10 μl PI in 1 × binding buffer for 15 min (Beyotime). Cells were visualized with the Leica SP8× Inverted Meta confocal microscope (Leica, Germany). The image was processed with the LSM image software (Leica).
Statistical analysis
SPSS 20.0 (IBM, Microsoft, USA) was used for all cell and animal experimental data, which were expressed as the mean ± se. The difference between groups was analyzed using unpaired Student t-tests and ANOVA tests for multiple group comparison. P < 0.05 was set statistical significance. All experiments were repeated at least 3 times independently.
Rat model with chronic olanzapine treatment demonstrated significantly body weight gain as well as abnormal glucose metabolisms
To explore patients’ metabolic syndromes in obese during olanzapine maintenance treatment [9], we generated rat models to mimic the weight gain phenomenon with long-term (8 weeks) olanzapine treatment. Initially, the average body weights of rats were 226.3 g ( ± 9.01) and 228.1 g ( ± 8.61), showing no significant difference between the control group and the olanzapine group. After treatments with olanzapine of 1.5 mg/kg (similarly clinical dosage for patients) [9], our results demonstrated that the mean weight gains of rats during olanzapine treatments were significantly progressively increased from week 1 till week 8 (endpoint of olanzapine treatment). There were 7.1%, 12.6%, 11.8%,14.1%, 14.0%, 14.1%, 14.6% and 17.5% substantially increases from week 1 to week 8, respectively (Figure 1) (p<0.05), suggesting that our rat models could fully mimic abnormal weight gain phenomenon during chronic olanzapine treatment in clinic.
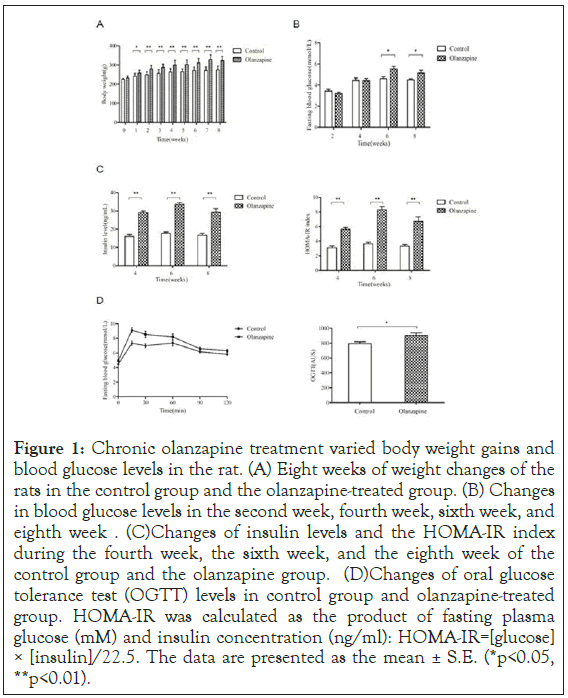
Figure 1: Chronic olanzapine treatment varied body weight gains and blood glucose levels in the rat. (A) Eight weeks of weight changes of the rats in the control group and the olanzapine-treated group. (B) Changes in blood glucose levels in the second week, fourth week, sixth week, and eighth week. (C) Changes of insulin levels and the HOMA-IR index during the fourth week, the sixth week, and the eighth week of the control group and the olanzapine group. (D) Changes of oral glucose tolerance test (OGTT) levels in control group and olanzapine-treated group. HOMA-IR was calculated as the product of fasting plasma glucose (mM) and insulin concentration (ng/ml): HOMA-IR=[glucose] × [insulin]/22.5. The data are presented as the mean ± S.E. (*p<0.05, **p<0.01).
We further examined the fasting blood glucose and insulin levels of rats both groups using similar ELISA assay kits, and the insulin resistance index was calculated as well. Our results showed that the blood glucose levels were 21% (week 6, 5.54 ± 0.85) and 15% (week 8, 5.14 ± 1.06) in olanzapine treatments higher than those of the control group (week 6, 4.59 ± 0.76) (p=0.003, p<0.01) and (week 8, 4.47 ± 0.42) (p=0.036, p<0.01) respectively. Similar effects on insulin levels after the olanzapine treatments in olanzapine group. The insulin levels were 81% (week 4, 29.1 ± 3.66) higher than that of the control group (week 4, 16.1 ± 4.27) (p=0.000, p<0.01). In week 6 and 8, the mean insulin levels were 89% (week 6, 33.7 ± 3.76) 75% (week 8, 29.3 ± 7.57) higher in olanzapine group were than those of the control group (week 6, 17.8 ± 2.66) (p=0.000, p<0.01) and (week 8, 16.7 ± 4.40) (p=0.000, p<0.01), respectively (Figure 1).
After performing OGTT assay, we also found that glucose metabolism was abnormal during olanzapine treatment as well (Figure 1). The area under the OGTT curve in the olanzapine- administered group (903.4 ± 126.3) was 8% more than that in the control group (794.3 ± 92.9), indicating that the glucose tolerance was significantly impaired after olanzapine administration (p<0.05).
Consistent with our previous study, we detected the insulin resistance index (HOMA-IR index) and found that values of HOMA-IR index were also significantly increased by 81% in week 4 (5.64 ± 0.96), 128% in week 6 (8.31 ± 1.66) and 104% in week 8 (6.72 ± 2.30) in olanzapine treatments than those in the control group (week 4, 3.11 ± 0.90), (week 6, 3.64 ± 0.86) and (week 8, 3.30 ± 0.90), respectively (Figure 1), (p<0.01). These results suggested that our rat model was successfully generated as insulin resistance showing abnormal obese by chronic olanzapine treatments in clinical dosage.
Apoptotic pathway activated in obese rat model and 3T3- L1 cells after olanzapine treatment
Previous studies highly postulated that substantial weight gains were correlated with adipocyte apoptosis in insulin resistance [5-7]. Our group also demonstrated that overweight of rodent insulin resistance model was partially owing to inflammation activation [9]. These reports implied a correlation between olanzapine- induced obese and apoptosis in the adipocyte. We then examined related factors activated in proliferation and apoptotic pathways in white fat tissue from our olanzapine and control rats. Consistent with the study by Lu et al. [22], our results also showed virtually stronger phosphorylated p-AKT and p-PI3K signals (Figure 2). Densitometry analyses observed significant more p-AKT by 167% increase and more p-PI3K by 174% increase from chronic olanzapine treatments compared to controls, respectively. These results implicated significant activations in adipocyte proliferation after chronic olanzapine administration in clinical dose.
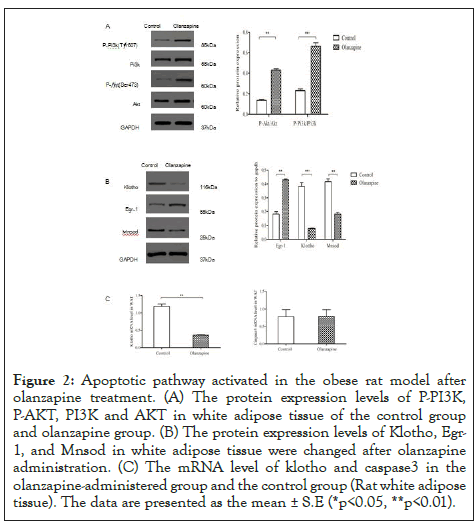
Figure 2: Apoptotic pathway activated in the obese rat model after olanzapine treatment. (A) The protein expression levels of P-PI3K, P-AKT, PI3K and AKT in white adipose tissue of the control group and olanzapine group. (B) The protein expression levels of Klotho, Egr- 1, and Mnsod in white adipose tissue were changed after olanzapine administration. (C) The mRNA level of klotho and caspase3 in the olanzapine-administered group and the control group (Rat white adipose tissue). The data are presented as the mean ± S.E (*p<0.05, **p<0.01).
Our proteome data showed strong altered expressions in anti-aging gene Klotho and ROS scavenge gene MnSOD (data not shown) after chronic olanzapine treatments. We then sought to check these protective factors to examine whether they would be activated when adipocyte proliferation increased. To our surprise, protein levels of Klotho and the counter-apoptotic factor, MnSOD were all virtually decreased (Figure 2) and densitometry analyses showed that they were decreased by 71% and 62% in white adipose tissues in the olanzapine group (p<0.05) compared to the control group. Consistent to our previous study [23], the pro-inflammatory factor Egr-1 was examined virtually strongly activated and densitometry results showed a significant increase in its protein levels by 118% than control(p<0.05). These results were suggesting that significant adipocyte proliferation was not owing to pro-proliferation gene activation during olanzapine treatments in clinical dose.
We then sought to check whether apoptotic pathway was triggered since the anti-aging gene and antioxidant enzyme MnSOD decreased after olanzapine administration. Equivalent with this postulation, the active form of the pro-apoptotic enzyme, caspase-3 was virtually substantially incremented measured by 73% increase compared to those in control white adipose tissues(p<0.05) after densitometry analysis (Figure 3). To rule out the active caspase-3 owing to gene upregulation, our quantitative PCR data showed there was no significant difference between olanzapine and control rat adipocytes (Figure 2). These results were consistent with the significant increase in inflammation regulator Egr-1 [24] (Figure 2) from current and previous studies, suggesting that inflammation involvements in obese after olanzapine chronic treatments which were also along with our previous work [9].
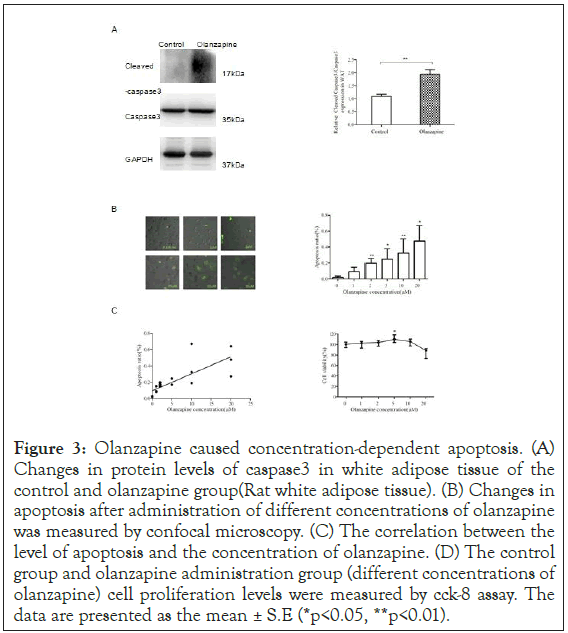
Figure 3: Olanzapine caused concentration-dependent apoptosis. (A) Changes in protein levels of caspase3 in white adipose tissue of the control and olanzapine group (Rat white adipose tissue). (B) Changes in apoptosis after administration of different concentrations of olanzapine was measured by confocal microscopy. (C) The correlation between the level of apoptosis and the concentration of olanzapine. (D) The control group and olanzapine administration group (different concentrations of olanzapine) cell proliferation levels were measured by cck-8 assay. The data are presented as the mean ± S.E (*p<0.05, **p<0.01).
Adipocyte proliferation was a feedback response owing to apoptosis activation after chronic treatments of olanzapine in clinical dose
Both adipocyte proliferation and inflammation-apoptosis were activated significantly from rat model after treatments with olanzapine in clinical dose, we then proposed that substantial elevated incrementing obese was ascribed to olanzapine initiating apoptosis. This obese could be the body's feedback response in a protective mechanism by which body activates the process of adipocyte proliferation after sensing apoptosis by olanzapine.
To confirm whether this apoptotic activation pathway indeed induced by olanzapine treatments, we first used 3T3-L1 adipocyte to testify further that olanzapine actually induced apoptosis reactions as observed in ANNEXIN V staining (Figure 3). Confocal imaging assay, Leica SP8x); and could prompt severe apoptotic consequences in a dose-dependent manner. Pearson’s correlation analysis showed that there was a significantly positive association between the concentrations of olanzapine and the counts in apoptotic adipocytes (Pearson’s correlation test, r=0.775, p=0.000).
In the meantime, our proliferation assays demonstrated that cell viability counts of 3T3-L1 adipocytes were growing along with olanzapine concentrations and reached significantly more than saline control in clinical dose (5 uM), increasing by 10% (p<0.05) (Figure 3). However, the growing rates of 3T3-L1 were inhibited after higher concentrations of olanzapine treatments from 10 to 20 uM, suggesting that severe apoptotic reactions dampen adipocyte proliferation rates in a larger dose to olanzapine than that in clinical applications. We further confirmed that the p-AKT and p-PI3K levels were virtually activated substantially in 5 uM olanzapine treatment (Figure 4a left panel). The densitometry results illustrated increases of p-PI3K and p-AKT levels by 50% and 80% (Figure 4a right panel) respectively, the same results as seen in our obese rat model of olanzapine treatment in clinical dose. Our data demonstrated that 5 μM of olanzapine elicited apoptosis reaction with clear downregulation of 77% klotho gene expression (Figure 4b left panel). The western blotting assay showed a virtually substantial decrease of klotho protein levels (Figure 4c left panel) by 51% after densitometry assay compared to saline-treated control 3T3-L1 adipocytes (Figure 4c right panel). Immunofluorescence also showed a decrease in Klotho expression after 5 uM olanzapine administration (Confocal imaging, Figure 4d). Consistent with results showing in our rat model, MnSOD protein level decreased virtually (Figure 4c left panel) by 26% after densitometry analysis (p<0.01, Figure 4d right panel); and substantially activated pro- inflammatory factor, Egr-1 (Figure 4c left panel) by 118% after densitometry analysis (p<0.05, Figure 4d right panel). Moreover, levels of active caspase 3 were increased in 5 uM olanzapine-treated 3T3-L1 adipocytes (Figure 4e left panel) by 117% increase after densitometry analysis compared to that in control group (p<0.01, Figure 4e right panel). These results implicated that the clinical dose of olanzapine could promote the apoptotic process and then lead a feedback response in adipocyte proliferation to cause abnormal body weight gain during chronic olanzapine treatment significantly.
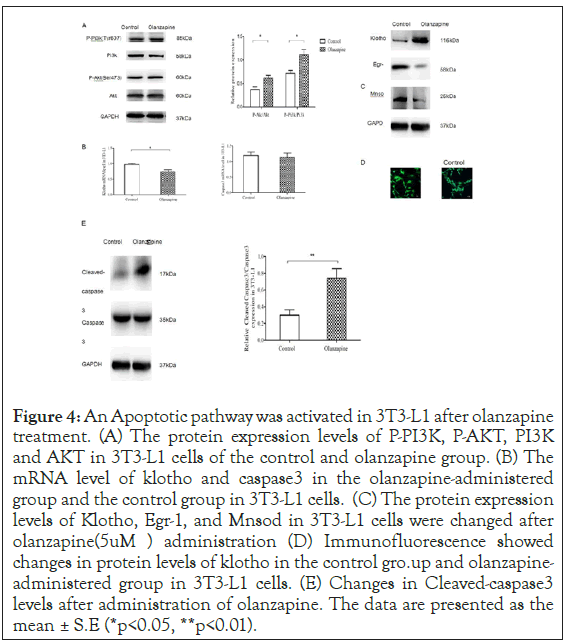
Figure 4: An Apoptotic pathway was activated in 3T3-L1 after olanzapine treatment. (A) The protein expression levels of P-PI3K, P-AKT, PI3K and AKT in 3T3-L1 cells of the control and olanzapine group. (B) The mRNA level of klotho and caspase3 in the olanzapine-administered group and the control group in 3T3-L1 cells. (C) The protein expression levels of Klotho, Egr-1, and Mnsod in 3T3-L1 cells were changed after olanzapine (5uM) administration (D) Immunofluorescence showed changes in protein levels of klotho in the control gro.up and olanzapine- administered group in 3T3-L1 cells. (E) Changes in Cleaved-caspase3 levels after administration of olanzapine. The data are presented as the mean ± S.E (*p<0.05, **p<0.01).
Growth and apoptosis in adipocytes induced by olanzapine were restored to normal by rKlotho in 3T3-L1 cells
Current results demonstrated that olanzapine could stimulate adipocyte proliferation by a feedback response of apoptosis through klotho-MnSOD cascade. To further confirm this hypothesis, we applied recombinant human klotho in 5 uM olanzapine-treated 3T3-L1 adipocytes. Our results demonstrated that rKlotho (1 μg/ ml) could reduce cell proliferation to an average level compared to 5 uM olanzapine treatment only (p<0.05, Figure 5a). In the meantime, the activated apoptosis by 5 uM olanzapine was apparently weakened by rKlotho application from confocal imaging (Figure 5a left panel), and rKlotho could significantly reduce the apoptotic rate to 8% (p<0.05, Figure 5b). Flow cytometry also showed the same results as significantly activation of cell apoptosis by 15% after olanzapine (5 μM) administration; however, the apoptosis level restored to standard cell culture less than 13% after application of rKlotho (p<0.05, Figure 5c).
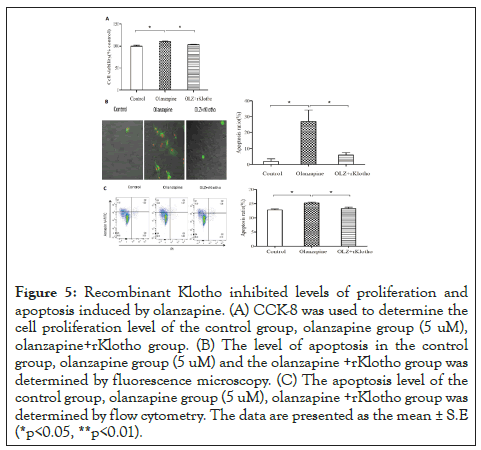
Figure 5: Recombinant Klotho inhibited levels of proliferation and apoptosis induced by olanzapine. (A) CCK-8 was used to determine the cell proliferation level of the control group, olanzapine group (5 uM), olanzapine+rKlotho group. (B) The level of apoptosis in the control group, olanzapine group (5 uM) and the olanzapine +rKlotho group was determined by fluorescence microscopy. (C) The apoptosis level of the control group, olanzapine group (5 uM), olanzapine +rKlotho group was determined by flow cytometry. The data are presented as the mean ± S.E (*p<0.05, **p<0.01).
These results further proved our hypothesis that excessive adipocyte proliferation leads to obese partially due to a feed-back pathway by apoptosis activation in chronic olanzapine treatments. Most interestingly, the dosage application of olanzapine accounts for favoriting the adipocyte proliferation or apoptotic consequence (Figure 6).
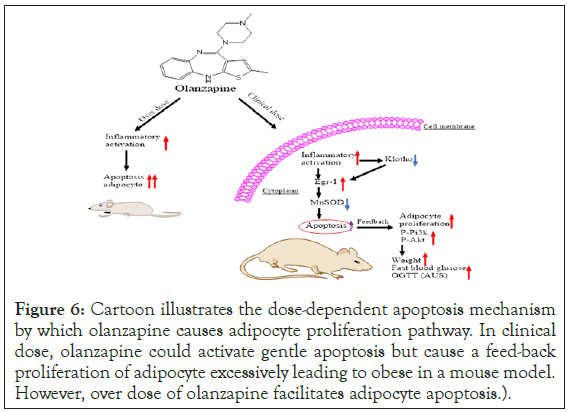
Figure 6: Cartoon illustrates the dose-dependent apoptosis mechanism by which olanzapine causes adipocyte proliferation pathway. In clinical dose, olanzapine could activate gentle apoptosis but cause a feed-back proliferation of adipocyte excessively leading to obese in a mouse model. However, over dose of olanzapine facilitates adipocyte apoptosis.
lanzapine is the first line antipsychotics but causes metabolic syndrome including significant insulin resistance and obese eventually leading to diabetes, which impair patient’s adherence to maintenance treatment [24]. The current study investigated the mechanism of substantial adipocyte proliferation as a feedback response induced by apoptotic activation during chronic treatments of olanzapine in clinical dose. Our results demonstrated that there were over-reacted proliferation cascades showing significant increases of phosphorylated AKT and PI3K in adipose tissues of chronic olanzapine-treated rats. Interestingly, our results showed extensively active apoptotic pathways via inflammation regulator Egr-1 activation and downregulation in antiaging factor, Klotho. We further proved our hypothesis using adipocyte cell line 3T3 L1 systematic experiments, which showed significantly elevated cell proliferation only in the clinical dose of olanzapine treatments. Two folds more than the clinical dose of olanzapine caused apoptosis mainly rather no feedback responses in cell proliferation. This is first-time observing olanzapine treatments causing apoptotic activation in adipose cells in a dose-dependent manner (Figure 6).
The previous study examined general insulin-secreting cell death in both hamster and mouse pancreatic beta cell lines [25]. However, the 100 uM of olanzapine treatments used in these experiments to these cell lines were exceedingly much higher than the ranges (around 5 uM of olanzapine in clinical dose) usually prescribed to treat mental health problems [26]. The current study showed thats olanzapine could cause adipocyte apoptosis in a far lower range of dose. There was even a balance in adipocyte proliferation and apoptosis in olanzapine treatments in the clinical dose range. This balance would incline toward adipose cell proliferation in clinical dose but fail to keep adipocyte proliferation when the dose was over 2-fold more than clinic amount (Figure 3). The minute dose of olanzapine activated apoptosis in adipose cell suggesting that adipocyte might be more sensitive than the pancreatic beta cell to this medication. This might be a reason for earlier overweight consequence than the later outcome of insulin resistance in olanzapine clinic observations [27].
The current study was indeed agreed with several studies showing apoptotic cascade activations in adipose cells correlated to obesity [5]. Adipocyte apoptosis was significantly associated with decreased levels of klotho protein, partially due to the decreased antioxidant enzymes MnSOD, which were consistent with other studies by Yoon et al. [28] showing that Klotho-induced cAMP could up-regulate MnSOD in HUVEC [29]. However, olanzapine could cause apoptosis outcomes in other types of cells as well. For instance, Bilgic et al. demonstrated that olanzapine could produce a significant increase in apoptosis of hepatocytes [30]. Other studies showed that olanzapine could inhibit the growth of glioma cells and induce autophagy and apoptosis of glial cells in vitro and in vivo [31]. Nonetheless, olanzapine had shown significant protective effects on nerve cells via inhibiting neuronal apoptosis through the GSK-3β-related pathway [32]. Varied feats of olanzapine on different cell types could explicate various metabolic syndromes such as overweight, dyslipidemia, and glucose intolerance [33].
A recent study investigated the genetic mechanism in a mouse model treated with olanzapine and reported that olanzapine- induced hyperphagia leading to excess weight gain due to blocking activity in 5-HT 2C receptor [33]. This study did not recapitulate the overweight gain phenomenon in its male mice counterpart. Although there were a bunch of assumptions that excess, overweight adverse effects were owing to the high affinity of olanzapine to serotoninergic, dopaminergic, or histaminergic receptors, the exact mechanism is still in debate. Some study did show overweight gain related to gender and age [34]. Most clinics observed fastest weight gain occurs in the first six months after starting an antipsychotic without gender preference [26]. To align with these clinic observations, our study showed that both female and male rats gained fast body weight after acute olanzapine treatments (Micro CT imaging data in our unpublished manuscript).
Current study proposed that inflammation-apoptosis induced a feedback adipocyte proliferation leading to obesity during olanzapine chronic treatments. Klotho, an anti-aging element could be a player involved in this adipocyte apoptosis proliferation balance in clinic dose of olanzapine administration. Although our results currently limited in vitro cell study, this piece of work might further prove that klotho could be a new target for improving the metabolic side effects of olanzapine.
This research was funded by funds from the National Natural Science Foundation (No.:81573509) granted to Weiyong Li and a Xinxiang Medical University endowment (No.: 505182) granted to Peng,S.
Conception and design: Yifan Lv and Shiyong Peng; Design and performance of experiments: Yifan Lv, Shouqin Liu, Jia Tong, Shihong Li, and Ni Yang; Composition of the manuscript: Shiyong Peng, Yifan Lv, Weiyong Li, Zhongjian Zhang; Data analysis and interpretation: Yifan Lv, Shouqing Liu, Ni Yang, Shihong Li, Wenqiang Li.
Citation: Lv Y, Liu S, Tong J, Zhang Q, Zhang Z, Peng S, et al. (2020) Atypical antipsychotic olanzapine induces obese via an apoptotic feedback pathway. J Clin Exp Ophthalmol. 10:453. DOI: 10.35248/2161-0495.20.10.453
Received: 20-Aug-2020 Accepted: 03-Sep-2020 Published: 10-Sep-2020 , DOI: 10.35248/2161-0495.20.10.453
Copyright: © 2020 Lv Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.