Autism-Open Access
Open Access
ISSN: 2165-7890
ISSN: 2165-7890
Review Article - (2024)Volume 13, Issue 4
Despite the potentially important roles of diet and nutrition to overcome symptoms associated with Autism Spectrum Disorder (ASD), the evidence to support these roles is widely perceived by the public and health professionals as being inconsistent. Food selectivity may further result in nutritional deficiency and the impact could be severe. Therefore, adequate nutrition should be provided to complement nutritional deficits in children with ASD. The most documented GI symptoms include chronic diarrhea, excessive gas, abdominal discomfort and distension, constipation, gastro-esophageal reflux, and food intolerances. Because of these symptoms, dietary intake and nutrient adequacy was found to be challenging. In this review, we present the nutritional deficiency and possible dietary solution to minimize ASD associated symptoms. The interaction of dietary nutrient during digestion and absorption with the physiological and metabolic functions, along with proper supplementation of nutrient are also discussed. Positive results of several studies suggest that a comprehensive nutritional and dietary intervention is effective at improving nutritional status, non-verbal IQ, autism symptoms, and other symptoms in most individuals with ASD.
Children; Autism; Nutrients; Diet; Metabolic functions
Throughout the world, there are growing concerns about developmental, behavioral, social and emotional wellbeing of children. Autism Spectrum Disorders (ASD) are characterized by impaired social and communication interactions as well as restricted, repetitive interests and behavior. Autism spectrum disorder is defined by two core symptoms: A deficit in social communication and the presence of repetitive behaviors and/or restricted interests. The reported prevalence of ASD in South Asia ranged from 0.09% in India to 1.07% in Sri Lanka that indicates up to one in 93 children have ASD in this region. Alarmingly high prevalence (3%) was reported in Dhaka city. Maenner, Shaw reported, across all 11 sites of USA, ASD prevalence was 18.5 per 1,000 (one in 54) children aged 8 years for 2016. The percentage of children aged 3-17 years diagnosed with a developmental disability increased from 16.2% in 2009-2011 to 17.8% in 2015-2017, specifically, diagnoses increased for Autism Spectrum Disorder (ASD) (1.1% to 2.5%) [1].
The Gastro-Intestinal (GI) problems, most commonly reported in autism, are chronic constipation, abdominal pain with or without diarrhea, and encopresis as a consequence of constipation. Food selectivity may further result in nutritional deficiency and the impact could be severe. Therefore, adequate nutrition should be provided to complement nutritional deficits in children with ASD. The purpose of this review was to determine potential risk factors for under-nutrition among autistic children by systematically reviewing the association between ASD and nutrient metabolism. These included the interaction between dietary nutrient, metabolism, absorption and resulting deficiency of certain macro-nutrient and micronutrients and certain phytochemicals [2].
Macro nutrients
Carbohydrates: Gastrointestinal hormones synthesized in functionally distinct populations of entero-endocrine cells and neurons play diverse roles in regulation of energy intake, nutrient absorption, and nutrient disposal.
A markedly increased prevalence of inadequate macronutrient intake i.e. carbohydrates (84.09% vs. 101.24%) was detected in the ASD group compared with the normal children, whereas Levy, Souders found that children with ASD consumed average amounts of calories and carbohydrates [3].
Some studies found abnormalities in carbohydrate digestion and absorption could explain some of the gastrointestinal problems in a subset of ASD patients. Kushak, Lauwers reported lactase deficiency in autistic children, and Lactase deficiency not associated with intestinal inflammation or injury is common in autistic children. Similarly, iIleal transcripts encoding disaccharidases and hexose transporters were deficient in children with autism, indicating impairment of the primary pathway for carbohydrate digestion and transport in enterocytes. Expression levels of disaccharidases and transporters were associated with the abundance of affected bacterial phylotypes resulting gastrointestinal disturbances. Likewise, studies showed dysregulation of glucose metabolism may be related to impaired adenosine triphosphate production and/or storage, or to some mitochondrial defects. A study found significantly decreased levels of ileal sucrose isomaltase, maltase glucoamylase, and lactase mRNA in children with ASD and gastrointestinal symptoms when compared to typically developing children who also had similar gastrointestinal symptoms.
The Keto-Diet (KD) is a proven metabolic therapy for medically intractable epilepsy, but there are only limited data for its use in ASD. In studies, autism was found to be treated with a glutenand casein-free KD (fats composed mostly of MCTs). Similarly, metabolic shift from the utilization of glucose to utilization of ketone bodies as a primary source of energy. Likewise, the patient’s behavior and intellect improved when on the KD. Investigator found some positive change of the Gluten-Free and Casein-Free (GFCF) diet on some (but not most) outcome measures. Citrulline levels increase with gluten-free diet and with improvement of enteropathy and citrulline levels are also inversely correlated with severity of intestinal malabsorption disease (i.e., coeliac disease) and Inflammatory Bowel Disease (IBD) such as Crohn's disease. Similarly, autistic child with Celiac disease, a GF diet would be necessary and beneficial lifestyle change, but may not affect the core autism symptoms [4].
Protein: The characteristics of proteins that influence their interaction with the GI tract in a source-dependent manner include their physico-chemical properties, their amino acid composition and sequence, their bioactive peptides, their digestion kinetics and also the non-protein bioactive components conjugated with them and post-translational modifications such as glycosylation and phosphorylation. Protein synthesis is inhibited when there is a lack of only one amino acid in the diet. Several studies showed protein intake is significantly low among ASD children.
In autistic children, essential amino acid (leucine, isoleucine, phenylalanine, methionine and cystine) and non-essential amino acid (hydroxyproline, serine and tyrosine) significant lower level than controls. Along with which, some plasma amino acids except for glycine and glutamic acids, while phosphoserine were increased with normal serum levels of urea, ammonia, total proteins, albumin and globulins (alpha 1, alpha 2, beta and gamma).
Decreased levels of plasma tryptophan isoleucine, phenylalanine and tryptophan along with slightly increased serine, and slightly decreased tyrosine and phenylalanine was noted by Adams, Audhya. They hypothesize that this is due to decreased protein intake or impaired protein digestion, and postulated that the latter hypothesis is more likely because most essential amino acid levels were normal. Likewise, found dysregulated amino acid metabolism (lower levels of aspartic acid, glycine, β-alanine, tryptophan, lysine and proline with significantly higher asparagine), high ammonia and oxidative stress were prevalent among autistic children. Low levels of certain plasma amino acids in children with ASD suggest both an impaired capacity for protein digestion as well as increased passage of dietary peptides into systemic circulation by way of compromised intestinal integrity. Finally, certain plasma amino acids either serve as neurotransmitters (e.g., glutamate) or serve as precursors (e.g., tryptophan and tyrosine) for important neurotransmitters (e.g., serotonin and dopamine). Perturbations in these systems are common in children with autism and contribute significantly to autistic symptoms. Similarly, Adams, Audhya reported lower level of taurine, which could be due to an impairment of the conversion of methionine to cysteine to taurine, but it could also be due in part to increased wasting of taurine in the urine. Similar another study, which also found low plasma taurine in children with autism, although that study found a greater difference [5].
Some studies have also shown elevated levels of certain plasma amino acids, such as glutamic acid, aspartic acid and taurine, while others have shown decreased levels of certain amino acids, especially glutamine. Increased glutamatemia may be dietary in origin or may arise endogenously for several reasons, among others, metabolic derangements in glutamate metabolism perhaps involving vitamin B6, defects or blockage of the glutamate receptor at the neuronal compartment, or alterations in the function of the neurotransmitters transporters. However, trend for children with autism who were on restricted diets to have an increased prevalence of essential amino acid deficiencies and lower plasma levels of essential acids including the neurotransmitter precursor’s tyrosine and tryptophan than both controls and children with autism on unrestricted diets. A recent study discovered that CPEB4, a molecule that regulates protein synthesis, is impaired in most cases of autism, which regulates protein synthesis that is important for long-term memory formation possibly through regulating local protein synthesis in neurons.
Specific products of proteolytic fermentation such as hydrogen sulfide, ammonia, and p-Cresol take place in case of incompletely digested dietary proteins that have a negative effect on gut health. Similarly, several studies indicate that increased levels of protein in the colon leads to increased levels of proteinmetabolizing (putrefactive) bacteria, reduced levels of fiberfermenting (saccharolytic) bacteria, and reduced bacterial diversity as well as increased levels of putrefactive metabolites in the colon. These metabolites have been associated with inflammatory bowel diseases such as ulcerative colitis. Therefore, the consumption of dietary proteins that are difficult to digest by the reduced digestive capacity of the fragile ASD intestine results in elevated levels of peptides in the gut. These aberrant peptides have the potential to negatively affect gut barrier integrity, feed proteolytic bacteria that produce harmful byproducts, and set off an aberrant immune response, either directly or through the promotion of bacterial dysbiosis. Similarly, if a proper balance of the amino acids is not received protein synthesis will cease when the amino acid in the lowest concentration (limiting amino acid) is exhausted. This is known as the “all or none” law. So, In case of ASD children, treatment option for ASD children with gastrointestinal symptoms is to provide diet with more digestible sources instead indigestible proteins [6].
Fats and fatty acid: The long chain omega-3 fatty acid, Docosahexaenoic Acid (DHA), is a major lipid in the brain recognized as essential for normal brain function. Polyunsaturated Fatty Acids (PUFAs) are the major components of brain and retina, and are the essential fatty acids with important physiologically active functions Docosahexaenoic Acid (DHA, 22:6ω3) and Eicosapentaenoic Acid (EPA, 20:5ω3) are omega‐3 fatty acid with 22 carbons and 6 double bonds (22:6n‐3). Polyunsaturated Fatty Acids (PUFAs) in three major forms, and somehow related, developmental psychiatric disorders: Autism, attention deficit and hyperactivity disorder and psychosis.
Ooi, Weng conducted a 12-week open-label study of 1 g/day of omega-3 fatty acids in 41 children aged 7-18 years with ASD, and found significant improvements in the core symptoms and attention problems. Moreover, omega-3 fatty acids supplementation is effective for improving core and associated symptoms of ASD. Some clinical trials showed the beneficial effect of dietary ω‐3 PUFA supplementation on behavior in various neurodevelopmental disorders, including autism. However, Mankad, Dupuis conducted a randomized controlled 6-month trial of 1.5 g/day of omega-3 fatty acids or a placebo in 38 children aged 2-5 years with ASD, and found no evidence for the efficacy of omega-3 fatty acids on improving core symptoms. Similarly, PUFAs did not show evidence of effect in children and adolescents with ASD and the certainty of evidence as measured with the GRADE was low to very low, but trials with larger sample size are critically requested.
DHA metabolic process occurs via separate channeled carnitinedependent mitochondrial pathway. The acyl-carnitine pattern found in ASD patients is not consistent with any known genetic disorders of fatty acid oxidation and organic acid metabolism, electron transport chain or urea cycle dysfunction, or other inherited metabolic diseases. Recent studies suggest a reduced flux through the mitochondrial β-oxidation pathway along with non-consistent acyl-carnitine pattern in a subset of patients with ASD. Genetic defects of mitochondrial β-oxidation are a group of IEM caused by failure of a single mitochondrial enzyme of β- oxidation such as Short Chain Acyl-CoA Dehydrogenase (SCAD), Medium Chain Acyl-CoA Dehydrogenase (MCAD), Very Long Chain Acyl-CoA Dehydrogenase (VLCAD) or Long Chain 3-Hydroxyacyl-coa Dehydrogenase (LCHAD). Mitochondrial β-oxidation defects may be secondary to dysfunction of dependent processes, such as deficiencies of the carnitine fatty acid transporter system, or mitochondrial electron transfer flavoprotein system (multiple acyl-CoA dehydrogenase deficiency). The occurrence of developmental delay, autistic-like behavior or ASD in genetic defects of mitochondrial β-oxidation particularly VLCAD and LCHAD suggests that impaired mitochondrial β-oxidation may contribute to dysfunctional energetic metabolism in subsets of patients with ASD. Long Chain Acyl-CoA Dehydrogenase (LCAD) has been shown to be the mitochondrial enzyme responsible for the beta-oxidation of branched chain and unsaturated fatty acids [7].
Newer biomarkers i.e biomarkers of fatty acid metabolism, nonmitochondrial enzyme function, apoptosis markers and mitochondrial antibodies. Additional metabolic abnormalities in patient include alterations of TCA energy production, ammonia detoxification, reduced synthesis of omega-3 DHA, and abnormal cholesterol metabolism resulting to secondary abnormalities in dysfunction of fatty acid beta-oxidation is also reported in autism. Similarly Frye, Melnyk noted abnormalities in fatty-acid metabolism found in subgroup of children with ASD were secondary To Tricarboxylic-Acid Cycle (TCAC) abnormalities.
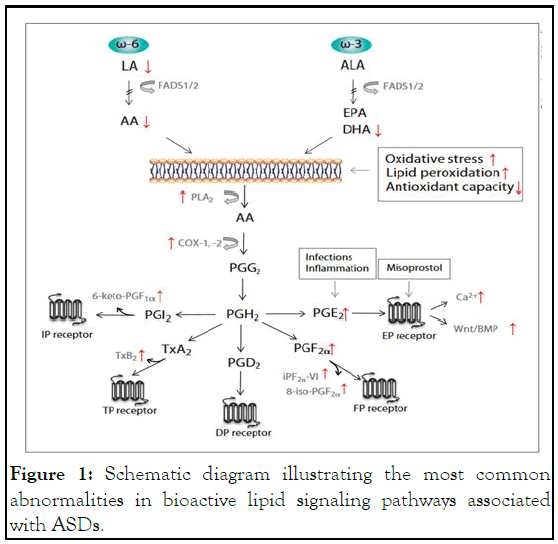
Figure 1: Schematic diagram illustrating the most common abnormalities in bioactive lipid signaling pathways associated with ASDs.
Dietary fiber: Dietary fiber acts on the gastrointestinal tract through several mechanisms, including increased fecal mass with mechanical stimulation/irritation of the colonic mucosa with increasing secretion and peristalsis, and the actions of fermentation byproducts, particularly short-chain fatty acids, on the intestinal microbiota, immune system and the neuroendocrine system of the gastrointestinal tract. Studies showed a positive correlation between fiber intake association with GI symptoms of abdominal pain, bloating, and constipation, flatulence, and diarrhea. Berding and Donovan reported higher intakes of vegetables, legumes, nuts and seeds, fruit, refined carbohydrates, and starchy vegetables, but lower intakes of sweets, was associated with lower abundance of Enterobacteriaceae, Lactococcus, Roseburia, Leuconostoc, and Ruminococcus. DP2, characterized by low intakes of vegetables, legumes, nuts and seeds and starchy vegetables, was associated with higher Barnesiellaceae and Alistipes and lower Streptophyta, as well as higher levels of propionate, isobutyrate, valerate, and isovalerate. Similarly, increasing evidence of altered fatty acid metabolic pathways may affect proper function of the nervous system and contribute to autism spectrum disorders. However, low level Of Short Chain Fatty Acids (SCFA's) was partly associated with increased probiotic use, and probably partly due to either lower production (less sacchrolytic fermentation by beneficial bacteria and/or lower intake of soluble fiber) and/or greater absorption into the body (due to longer transit time and/or increased gut permeability). Therefore, consideration of recommended fiber intake, when considering treatment of abdominal pain and constipation in children with ASD. Yasukawa, Inoue reported intake of Partially Hydrolyzed Guar Gum (PHGG) improves human stool form via regulating intestinal microbiota in diarrhea, i.e.,Irritable Bowel Syndrome Diarrhea (IBS-D)-like symptoms [8].
Micro nutrients
Vitamins: Children with ASD were more likely to consume vitamin/mineral supplements than typically developing children. For the vitamins, 20% lower biotin (p<0.001), and significant (p<0.05) lower levels of vitamin B5, vitamin E and total carotenoids, along with vitamin C was possibly slightly higher in the children with autism. Vitamin B6 (measured as the active form, P5P, in the RBC) had an unusually broad distribution in children with autism compared to controls. Compared to typically developing children, those with ASD have been reported to consume less folic acid, vitamin B6, vitamin A, vitamin B1, B3, B7, Vitamin B12 and vitamin D. The Micronutrient Survey showed severe anemia due to Zn, B12, folic acid deficiencies along with vitamins E, D, A, and folic acid.
Three studies have demonstrated that children with autism found nutritional supplementation (with vitamin methyl-B12, folinic acid, and trimethylglycine) is beneficial. The multi vitamin supplement increased the level of most vitamins, including vitamins B1, B3, B5, B6, biotin, folic acid and B12, while higher levels of vitamin B2 are needed in the supplement to affect blood levels. Similarly, double-blind, placebo-controlled study of high-dose vitamin C (110 mg/kg) found a reduction in autism severity. Different studies showed supplementing infants with Vitamin D might be a safe and more effective strategy for reducing the risk of autism.
Vitamin A: Children with autism have vitamin A deficiency than control along with vitamin D deficiency was also noted, and co-deficiency of vitamin A and D may exacerbate the symptoms of children with ASD. Similarly, serum retinol levels in children with ASD were significantly lower than in control children. Serum 5-HT levels in children with ASD were higher than in control children, which were correlated with symptom severity of children with autism. After vitamin A supplementation, the children with ASD exhibited significant improvement in autism symptoms. Serum retinol concentrations of children with ASD were significantly increased, and serum 5- HT levels were decreased.
Adams, Audhya reported that carotene levels improved, but were still somewhat low, so higher amounts are needed, and supplementation with carotenes and modest amounts of vitamin A did not significantly alter vitamin A levels; body only converting carotenes to vitamin A if vitamin A levels are low.
Bacteroidetes/Bacteroidales were the key taxa related to vitamin A, and it played a role in the changes in autism biomarkers. Interestingly, Liu, Liu found both CD38 and RORA mRNA levels significantly increased in ASD after the vitamin A injection, and the potential use of vitamin A treatment and the necessity of clinical trials using vitamin A in ASD were also illustrated by Megson in 2000. RA has been considered a transcriptional regulator that can shape synaptic plasticity in the brain, thereby accounting for learning and memory ability. Vitro study also demonstrated that RA can up regulate CD38 transcription levels; RA may regulate RORs (including RORA, RORB, RORR) through its retinoic acid receptors, which demonstrated the potential role of vitamin A in autism biomarker) Liu, Liu.
Vitamin B complex
Thiamine: Thiamine deficiency contributes to a number of conditions spanning from mild neurological and psychiatric symptoms (confusion, reduced memory, and sleep disturbances) to severe encephalopathy, ataxia, congestive heart failure, muscle atrophy, and even death. And the study showed beneficial effect of thiamine supplementation in Autism Spectrum Disorder (ASD) and other neurological conditions.
A relationship between thiamine status and the development of autism has been established, with thiamine supplementation exhibiting a beneficial clinical effect on children with autism. Thiamine may involve in autism via apoptotic factors (transcription factor p53, Bcl-2, and caspase-3), neurotransmitter systems (serotonin, acetylcholine, and glutamate), and oxidative stress (prostaglandins, cyclooxygenase-2, reactive oxygen species, nitric oxide synthase, the reduced form of nicotinamide adenine dinucleotide phosphate, and mitochondrial dysfunction).
Pyridoxine: The amount of vitamin B6 is moderately high compared to the RDA because in children with autism many B6- dependent biomarkers were known by research to be abnormally low, including glutathione and neurotransmitters. The finding of high vitamin B6 levels is consistent with low levels of pyridoxal-5-phosphate and low activity of pyridoxal kinase (i.e., pyridoxal is only poorly converted to pyridoxal-5-phosphate, the enzymatically active form. A study found that children with autism had high levels of plasma vitamin B6 presupplementation, and this finding was confirmed in a follow-up study, suggesting a metabolic imbalance in B6 [9].
Folic acid: The Folate Receptor-α (FRα), which is responsible for transportation of folic acid into the brain, might be blocked in Folate Receptor-α Autoantibody (FRAA)-positive patients or due to mitochondrial dysfunction or genetic mutations, and thus decrease CSF folate concentrations.
Randomized double-blind placebo-controlled trial has tested high-dose folinic acid for 12 weeks and found significant improvements in verbal communication. Since folinic acid may become increasingly used to treat ASD in the future, its safety should be further ensured.
Vitamin B12: Methyl-cobalamin is actively taken up by neurons, and it has been indicated for the treatment of nervous disorders through effective systemic or local delivery. The combination of methylcobalamin (mB12) with Low-Dose Folinic Acid (LDFA) and sapropterin, a synthetic form of tetrahydrobiopterin (BH4) have been studied in open-label trials while high-dose folinic acid has been studied in a double-blind placebo controlled trial. All of these treatments have the potential to positively affect folate, methylation and glutathione pathways. Similarly, supplementation of vitamin B12 might be of help for the reduced vitamin B12 level and elevated homocysteine levels and other metabolic processes in ASD induced by N2O exposure. The results showed that methyl B12 supplementation improved symptoms of ASD. In a rat sciatic nerve injury model, continuous administration of high doses of methylcobalamin improves nerve regeneration and functional recovery was noted.
Vitamin C: A single case report presents a child with autism spectrum disorder and food selectivity difficulties that resulted in severe vitamin C deficiency. Vitamin C is considered to be a vital antioxidant molecule in the brain. Intracellular ascorbate serves several functions in the CNS, including antioxidant protection, peptide amidation, myelin formation, synaptic potentiation, and protection against glutamate toxicity. Vitamin C levels in the autism group were initially above the neurotypical group, and the supplement raised those levels significantly with probably beneficial, as the children with autism initially had high oxidative stress, and the supplement significantly decreased the level of oxidative stress, probably in part due to the vitamin C in the supplement. Sensory motor scores indicating a reduction in symptom severity associated with the ascorbic acid treatment was noted [10].
Vitamin D: Vitamin D is a fat-soluble vitamin that plays an important role as neuroactive steroid that involves in the development of brain, bone metabolism and seems to have some anti-inflammatory and immune-modulating properties, which are also involved in multi biological activities, including cellular proliferation, differentiation, calcium signaling, neurotrophic, and neuroprotective actions. Optimal vitamin D status may contribute to improving behavioral pathophysiologies resulting from dysregulation of serotonergic neurotransmission.
Several studies reported significant differences in 25(OH) D concentrations between cases and controls. Furthermore, lower vitamin D levels were reported in subsets of patients with ASD compared to healthy controls. Vitamin D deficiency was higher in autism children compared to healthy children and supplementing infants with vitamin D might be a safe and more effective strategy for reducing the risk of autism, whereas some studies had also reported vitamin D supplementation had no effect on the primary outcome with limited and inconsistent effects in children with ASD.
Vitamin E: In vitro, and probably in vivo, vitamin E acts as an antioxidant, and the only significant lipid-soluble, chain breaking type of antioxidant present in human blood.
The neuro-pathological findings support the concept for the maintenance of the integrity and stability of biological membranes, and one postulate that it protects the phospholipids of biological membranes from peroxidation and maintaining of the other molecules in their correct oxidation state. Symptomatic vitamin E deficiency has been reported in genetic defects of the vitamin E transport protein and in mal-absorption complicating celiac disease. And Celiac Disease (CD) prevalence among autism was 2.62%, which is statistically significant higher to that reported in the Italian pediatric population. In experimental studies, vitamin E has been shown to attenuate toxic effects of β- amyloid and improve cognitive performance in patients with moderately severe neurological impairment. Treatment with α- tocopherol (2000 IU a day) reduces neuronal damage and slows the progression of the diseases, which indicates that the use of α- tocopherol may delay clinically important functional deterioration in autistic patients. Several studies have highlighted the damaging effects of UV radiation on the eyes. Roberts found that prolonged exposure to UV radiation increases the risk of cataracts and macular degeneration. Davidoff emphasized the importance of protecting the eyes from invisible light, including UV radiation emitted by artificial light sources. The health and safety executive issued an information sheet stressing the potential ocular hazards of UV radiation. They recommend the use of protection, such as sunglasses or safety glasses labeled for 100%UV protection, to shield the eyes from the harmful effects of UV radiation. To mitigate the risk of photokeratitis due to exposure to unshielded halogen bulbs, proper shielding and barriers are crucial. Research by Pitts and Tredici demonstrated that sunglasses with adequate UV filtration effectively protected the eyes from UV radiation. Applying this principle to halogen bulbs, integral glass or plastic coverings can be employed to shield the bulbs and prevent direct exposure [11].
Vitamin K: Characterization of vitamin K-dependent proteins that are now known to play key roles in the central and peripheral nervous systems and vitamin K as an important nutrient for the nervous system. Specific neural effects of vitamin K overlap with key brain development aberrations, including those associated with autism. Furthermore, vitamin K protects against oxidative stress associated with toxic exposure. Research on a small sample of severely autistic children of Somali descent residing in the Minneapolis/St. Paul area of Minnesota were genotyped and found to have a higher than expected genetic substitution that results in reduction in the efficiency of the vitamin K cycle. There is now data to suggest that vitamin K has the potential to influence psychomotor behavior and cognition. Oral vitamin/mineral supplementation is beneficial in improving the nutritional and metabolic status of children with autism, including improvements in methylation, glutathione, oxidative stress, sulfation, ATP, NADH, and NADPH; both biotin and vitamin K are made by beneficial intestinal flora. The future possibility of influence of vitamin K on psychomotor behavior and cognition could play a role in the ASD symptoms [12].
Minerals: Large proportions of ASD children did not meet national recommendations for daily intake calcium, zinc, iron etc in compared with controls and/or reference ranges of healthy children.
Children with autism have lower concentrations of magnesium (serum, hair, and nail), iodine (hair), chromium (hair), and selenium (hair and nails). A study reported children with ASD aged 4 to 8 years consumed significantly less Zn; and those 9 to 11 years consumed less phosphorous. Few children in either group met the recommended intakes for calcium and potassium. Specific age groups consumed excessive amounts of sodium, folate, manganese, zinc, selenium, and copper, and special consideration should be given to calcium.
Calcium: Compared the dietary intake of children with ASD to that of children with normal children, they noted that large proportion of children do not meet national recommendation for daily intake of calcium. A cross sectional study conducted by NRC Egypt among autistic children of age group 7-9 years found that calcium were 50.89% and 68.9% of RDA respectively. Considering the role of calcium regulation in neurodevelopment and neuroplasticity, low calcium during early brain development could be a risk factor for adverse neurobehavioral outcomes. Children with ASD should be screened for vitamin D status with 25(OH) D levels and serum phosphorus levels and assessed for vitamin D and calcium intake. Supplementation should be implemented based on clinical judgment after evaluation of risk factors.
Iron: Deficiency of iron as well as anemia were more common in autistic compared to control children. The micronutrient survey showed severe anemia due to iron, Zn, deficiencies. Children with ASD are at risk for low SF, which has been associated with attention and sleep problems. Biomarkers such as hepcidin and transferrin receptor play an important role in iron absorption and transport into red blood cells. The reason for lower ferritin levels in children with autistic disorder is unclear. Autism and iron deficiency could be linked by a common underlying genetic mechanism that has not been identified yet. Alternatively, because iron is involved in brain monoamine systems, iron may influence autism through its effects on monoamine-dependent neurotransmission. As intestinal dysfunction was described in autism, impaired absorption might be a possible cause of iron deficiency. However, Iron supplementation of children with ASD is not recommended without documentation of parameters of iron status, and treatment should be monitored for laboratory response. Iron treatment can result in side effects, including constipation and gastrointestinal distress, and there is risk of toxicity with overdose [13].
Zinc and copper: Studies in children with autism found lower levels of zinc in plasma and RBC compared to neurotypical children. Similarly, a study of dietary intake of autistic children in China found that most had inadequate intake of zinc. Decreased zinc status in autism has been clearly established as a potentiator of oxidative stress. Zinc deficiency increases lipid peroxidation and free radicals in cell membranes, mitochondria, and other tissues. It also decreases the total glutathione, glutathione-S-transferase, vitamin E, and SOD levels. Copper is highly pro-oxidant and this is especially true of unbound copper; however, supplemental copper is rarely need in autism.
Even small doses of copper have been suggested to produce negative behavioral effects. Hypothetically, oxidative stress can decrease the clinical zinc retention; therefore, it seems that zinc is an essential constituent of copper-zinc SOD, which is a key antioxidant enzyme.
Dietary zinc alters neurological function from synapses to behavior, and identifies dietary zinc as a potential therapeutic agent in ASD. Autism caused by Shankopathies, having one intact copy of SHANK3 left, may benefit from zinc supplementation, as elevated zinc may drive remaining Shank3 into the Post-Synaptic Density (PSD) and may additional recruit Shank2, a second zinc-dependent member of the SHANK gene family. Infantile zinc- and magnesium-deficiency and/or toxic metal burdens may be critical and induce epigenetic alterations in the genes and genetic regulation mechanisms of neurodevelopment in the autistic children.
Magnesium: Infantile zinc- and magnesium-deficiency and/or toxic metal burdens may be critical and induce epigenetic alterations in the genes and genetic regulation mechanisms of neurodevelopment in the autistic children. When the Mg-B6 treatment was stopped, PDD symtoms reappeared in few weeks. Behavioral improvement observed with the combination vitamin B6-magnesium in PDD/autism is associated with concomitant modifications of Erc-Mg values. However another review reported no recommendation can be advanced regarding the use of B6‐Mg as a treatment for autism [14].
Phyto-chemicals: Antioxidant compounds possess several distinct mechanisms that enhance the glyoxalase pathway and function as neuroprotectantsss. Flavonoids are well-researched secondary plant metabolites that appear to be effective in reducing levels of oxidative stress and inflammation in neural cells. Autism Spectrum Disorders (ASDs) have been associated with brain inflammation as indicated by microglia activation, as well as brain expression and increased plasma levels of Interleukin-6 (IL-6) and Tumor Necrosis Factor (TNF), and the advantages of polyphenols in the treatment of autism disorders was reported. The flavone luteolin has antioxidant, antiflammatory, anti-allergy and neuroprotective properties. GI and allergy symptoms improved in about 75% of children, eye contact and attention in 50%, social interaction in 25% and resumption of speech in about 10%. Phyto-chemical exerts neuronal cytoprotective action possibly due to anti-oxidant action and could be efficacious in the management of autism [15].
The management of symptoms of ASD children requires a multi-disciplinary approach, which combines both nutritional and medical management. In this disorder, proper and adequate nutritional therapy is a critical component of the management of nutritional deficiency, GI symptoms such as chronic diarrhea, excessive gas, abdominal discomfort and distension, constipation, and/or behavior patterns. The results of our study demonstrated that a large proportion of ASD children have possible macro and micro nutrient deficiency, and certain symptoms can be minimized by supplementation.
Future studies using a larger sample size and measuring other behaviors associated with ASD are needed to investigate whether dietary intake may be a modifiable moderator of ASD symptoms. Furthermore, nutritional therapy for the ASD children is lifelong. Thus, registered dietitians and/or nutritionists trained in the area of metabolic disorders are an essential part to delivers care for those patients.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Koirala P (2024) Autism Spectrum Disorder (ASD) and Nutrition: A Review. Autism-Open Access. 14:399.
Received: 16-Apr-2020, Manuscript No. AUO-24-3898; Editor assigned: 21-Apr-2020, Pre QC No. AUO-24-3898 (PQ); Reviewed: 05-May-2020, QC No. AUO-24-3898; Revised: 22-May-2024, Manuscript No. AUO-24-3898 (R); Published: 19-Jun-2024 , DOI: 10.35248/2165-7890.24.14.399
Copyright: © 2024 Koirala P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.