Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Research Article - (2018)Volume 3, Issue 2
Beverages in aluminium cans are widely available, and for convenience, drinks are often consumed directly from the can. Cans are exposed to various environments during production, storage and shipping during which the lid may be contaminated with microorganisms. This study on can lid contamination was divided into three experiments to determine (1) the cleanliness of randomly collected cans, (2) the transfer rate from hands to can lids and (3) the survival of bacteria on lids during storage. Over 190 cans were tested for presence of ATP using a standard luciferase enzyme kit. Of the 194 randomly selected cans, 90 (46.39%) were in the dangerous unsanitary category, 60 (30.93%) were considered cautionary as far as sanitation while 44 (22.68%) were categorized as clean. In the second experiment, subjects handled and opened cans with hands inoculated with E. coli and greater than 50% transfer to wet cans lids and greater than 30% transfer to dry can lids was found. In the third experiment, inoculated can lids were found to harbor E. coli for up to 14 days.
Can lids; Bacterial contamination; Bacterial transfer; ATPase test
In 1810, Englishman Peter Durand patented the preservation of food in tin-coated iron cans then in 1813 two other Englishmen Brian Donkin and his brother-in-law John Hall bought Durand’s patent to set up the first commercial canning operation in London [1]. One year before the London commercial operation, Thomas Kennett started canning meats, fruits and vegetables in New York City. Although food had been sold in cans for many years, the first commercial can of beer was sold in Richmond, VA in January of 1935 and by the end of that year over 200 million cans of beer had been sold [2]. First introduced in 1965, aluminium beverage cans now make up about 75% of the beverage can market share and according to the aluminium can manufacturers association there are about 100 billion aluminium beverage cans produced in the US each year [3]. There are many different container types and materials used to package beverages, such as glass bottles, aluminium cans, and plastic bottles, all of which have the potential to become contaminated. With the popularity of beer and soft drinks, beverages stored in cans are extremely common. These cans are often transported and stored in packaged boxes. Cans are often packed and displayed with the tops uncovered. During storage and transportation, microorganisms may contaminate cans. Thus when drinking from a can, one’s mouth comes in direct contact with the can lid allowing possible transfer of microorganisms.
Published research is limited on bacterial presence, survival and transfer to can lids; however, Brook [4] reported that soda can tops had one of the highest levels of bacteria for a variety of surfaces humans are exposed to on a daily basis, including toilet seats, ATM key pads, bus poles, bathroom door handles, escalators and elevator buttons. Furthermore, Özkan [5] measured the amount of bacteria present on can lids before and after five different cleaning methods. Total aerobic bacteria recovered averaged 280, 29, 8.8, 21.2 and 3.2 CFU/ml for the uncleaned or cleaned with tissue, wet wipe, tap and soapy water, respectively. A study conducted on both food and beverage cans found that there was no correlation between the visual appearance of cleanliness on the tops of aluminium cans and the level of microbial contamination [6]. This study also reported that an effective way to clean the surface on the can lids was to rinse the can top and then wipe it with either a paper towel or a napkin.
A separate report compared slide-top, screw-top, squeeze-top, and straw-top water bottles [7]. Slide-top bottles were found to have 933,340 cfu/cm2, screw-top had 159,060 cfu/cm2, squeeze-top had 161,971 cfu/cm2, and straw top had 25.4 cfu/cm2 indicating the type of bottle had an effect on the number of bacteria present. This study implies there is a need for more information about bacteria on beverage cans.
Thus the present study objectives were to measure the
1. Presence of microorganisms on can lids found in public places;
2. Transfer of bacteria from handling and
3. Survival of bacteria on lids.
Experiment 1
Contamination level of randomly selected cans: One hundred and ninety-four beverage cans were randomly collected over a 7-week period from a variety of locations including retail refrigerated units, grocery shelves, and from the home. Each can was swabbed using moderate pressure around the inside rim of the can’s lid 3 times, above the lid in a streaking motion 3 times, and behind the lid in a streaking motion 3 times using the UltraSnap Surface adenosine triphosphate (ATP) testing swabs (Hygiena, Camarillo, CA). Extreme caution was taken to ensure that the swab did not touch any other surface.Samples were measured by snapping then squeezing the sample tube to mix the contents initiating the enzymatic reaction. The ATP swabs were then placed into a Hygiena EnSure ATP Luminometer, to detect the amount of light emitted which yields a relative light unit (RLU) value. According to the manufacturer a RLU between 0-10 is considered clean, 11-30 is considered cautionary, and any RLU over 30 is considered unsafe [8].
Experiment 2: Transfer of bacteria from hands to can lids
Bacterial inoculum: An Escherichia coli ampicillin-resistant strain with a fluorescent gene was used for the bacterial transfer and survival studies. A non-pathogenic E. coli strain JM109 was labeled with jellyfish green fluorescent protein according to the following protocol as described previously [9]. The competent bacterial cells were electroporated in a Gene Pulser II (Bio-Rad) with plasmid vector pGFPuv (ClonTech, Palo Alto, CA). Transformants were selected from isolated colonies grown on Luria-Bertani agar (LB) plates containing 100 g ampicillin/mL. The resulting ampicillin-resistant transformants emitted bright green fluorescence under UV light. The stability of GFP label in the E. coli strain was determined by streaking on trypticase soy agar (TSA) plates containing 100 g ampicillin/mL for several generations. The E. coli JM 109 culture was held in a -80°C freezer in vials containing tryptic soy broth (TSB) (Becto™, Becton Dickinson and Company, Sparks, MD, USA) supplemented with 20% (v/v) glycerol (Sigma, St. Louis, MO, USA). The frozen vial was thawed at room temperature prior to culturing. From this thawed vial, 0.1 mL of culture was transferred to 10 mL TSB (DIFCO™, Becton Dickinson and Company, Sparks, MD, USA)) containing 0.5% ampicillin (Sigma, St. Louis, MO, USA) in 2 loosely screw-capped tubes and then the tubes were incubated for 16 - 18 h at 37°C with vigorous shaking (Thermolyne Maxi-Mix III type 65,800, Barnstead/Thermolyne, Dubuque, IA). The second transfer was prepared from this first transfer culture by adding 0.1 mL from the first transfer tube to another fresh 10 mL TSB (DIFCO) with 0.5% ampicillin (Sigma), and again incubated for 16-18 h at 37°C with shaking. After incubation, the cells were harvested by centrifugation at 3000 rpm (1200 g) (IEC HN-SII Centrifuge, International Equipment CO., Inc., Needham Heights, MA, USA), then the pellet re-suspended in 10 mL of sterile peptone solution (0.1%) (Bacto peptone, Becton Dickinson) to obtain a population of approximately 6-7 log CFU/mL. Initial cell populations were verified by enumeration of the cells following surface plating in TSA containing 0.5% ampicillin (DIFCO™ Tryptic Soy Agar, Becton Dickinson and company Sparks, MD, USA) and incubating at 37°C for 24 h.
Experimental treatments
The two experimental treatments were 1) dry can lids and 2) wet can lids being touched and opened with inoculated hands. Each subject washed their hands with warm water and soap, let them dry and then 1mL of the E. coli inoculum was deposited in the center of their dominant hand. The approximate number of bacteria used per inoculation was 106 CFU/ml. The E. coli was spread onto hands and allowed to air dry for 30 s. Subjects then touched the top of an empty can with fingers and opened the can. This procedure was repeated with a can lid that had been wetted with one spray of a sterile water mist. A non-inoculated can lid was included as a control to verify that only fluorescently-labeled bacteria from hands were enumerated on can lids. Can lids were cut off the top of the can using a sterile knife and scissors then placed in a stomacher bag containing 20 ml of 0.1% peptone water and mixed for 30 s. To enumerate bacteria on subject’s hands the dominant hand was placed into a sterile stomacher bag with 20 mL of sterile 0.1% peptone and rinsed for 30 s, covering all fingers, palm, and back of the hand.
Enumeration was accomplished by taking 1 or 0.1 ml sample from the 20 ml stomacher bags after mixing then conducting serial dilutions in sterile 0.1% peptone water. Dilutions were surface plated in duplicate on TSA containing 0.5% ampicillin (DIFCO™ tryptic soy agar, Becton Dickinson and company Sparks, MD, USA) and incubating at 37°C for 24 h. After incubation, dilution plates with 25-250 colonies were counted under UV light (to illuminate fluorescent E. coli cells) the populations converted to colony forming units per can lid or hand (CFU/lid or CFU/hand). The % transfer of E. coli from hands to can lids was calculated using:
% transfer = CFU recovered from lids/CFU recovered from hands + CFU recovered from lids × 100
Experiment 3:
Survival of E. coli on can lids
The same bacterium (fluorescent E. coli) used in Experiment 2 was used for this experiment. Can lids were inoculated with ~106 cells of E. coli then sampled after 1, 7 and 14 days of storage at either room (22 ± 4°C) or refrigerated (4 ± 2°C) temperatures. Enumeration of bacteria on can lids was accomplished in the same way that was used for Experiment 2.
All three experiments were conducted as completely randomized designs and simple mean, standard deviation, minimum and maximum values were determined for treatment and where treatment effects were significant (P ≤ 0.05), means were separated for significance (P ≤ 0.05) using lsmeans in the Statistical Analysis System, SAS On Demand for Academics [10].
Experiment 1
Contamination level of randomly selected cans: Adenosine triphosphate is one of the primary energy carriers used in all living cells and present in nearly all organic matter. Microorganisms produce ATP in processes such as photosynthesis, storing energy as ATP metabolized from food and other energy sources. This study used ATP- bioluminescence (ATP-B) swabs to test the levels of contamination on the surface of aluminium can lids, as the presence of ATP can indicate the presence of bacteria. Of the 194 randomly selected cans, 90 (46.39%) had RLU readings of greater than 30 which is in the dangerous unsanitary category, 60 (30.93%) had RLU between 10 and 30 which are considered cautionary as far as sanitation and 44 (22.68%) had a RLU of <10 and were categorized as clean (Figure 1). The average RLU for all samples was 98.3 while the median was 27. The location from which the cans were sampled generally had no effect on cleanliness since 44 % of the cans taken from the home or retail setting were both determined to be dangerously unsanitary. Larson et al. [11] reported that while ATP-B monitoring of table tops and hands was a good indicator of sanitation, however the luminescence values did not correlate well with colony-forming units from the same surfaces. Conversely, of 252 food service establishment surfaces sampled, 96% of those that failed the ATP-B (RLU ≥ 200) test also failed the microbial plate count test (CFU>125/50 cm2) as well [12].
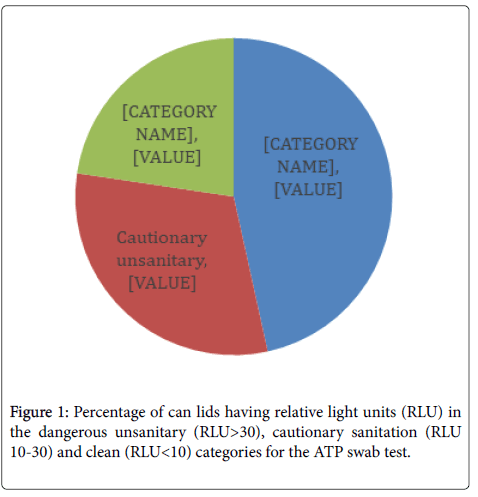
Figure 1. Percentage of can lids having relative light units (RLU) in the dangerous unsanitary (RLU>30), cautionary sanitation (RLU 10-30) and clean (RLU<10) categories for the ATP swab test.
Experiment 2
Transfer of bacteria from hands to can lids: Significantly more E. coli was transferred to wet can lids compared to dry lids when handled with inoculated hands (Figure 2). However, an average of over 4 logs/can lid were still recovered from dry lids from hands inoculated with 5 log cycles of E. coli. Since beverages in cans are often served cold with potential water condensation or from being held in ice slurry, can lids will potentially be wet. Patrick et al. [13] reported a 94 to 99% reduction in translocation of bacteria between contaminated surfaces by dry hands compared to wet hands. While the study by Patrick et al. [12] tested wetting hands and the current study evaluated wetting the surface before contact with the surface, both studies demonstrated greater bacterial transfer by wet surfaces. The increased transfer on wet surfaces was also demonstrated by Moore and Griffith [14] who found a 10,000 times increase in detection levels with wet surface swabbing techniques compared to dry techniques. This trend was also supported by Lopez et al. [15] who reported a 3.8% transfer of E. coli from stainless steel to fingertips with a 15-32% relative humidity (RH) but a 54% transfer at 40-65% RH.
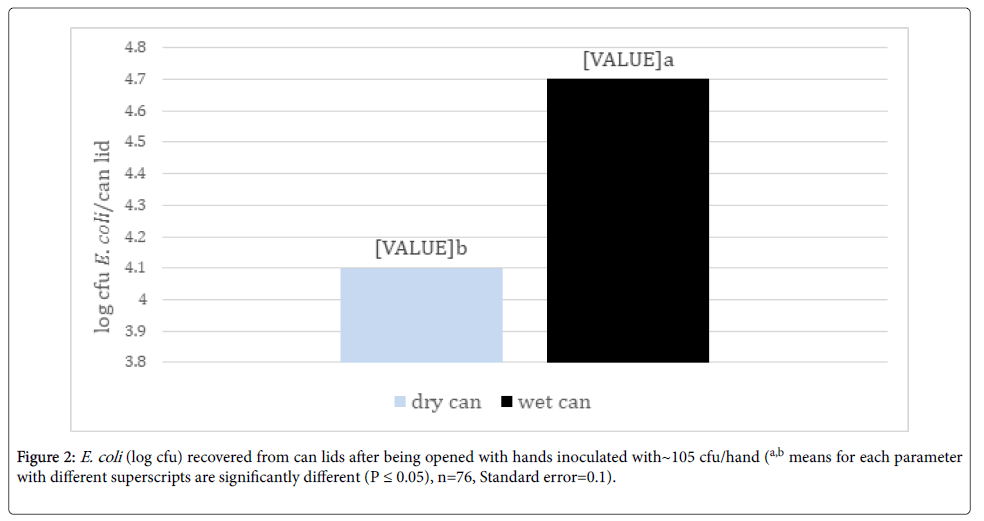
Figure 2. E. coli (log cfu) recovered from can lids after being opened with hands inoculated with~105 cfu/hand (a,b means for each parameter with different superscripts are significantly different (P ≤ 0.05), n=76, Standard error=0.1).
The percentage of E. coli transferred to wet can lids from inoculated hands was over twice that transferred to dry can lids (Figure 3). Keeratipibul et al. [16] supported these findings showing a 30% decrease in recovery of bacteria from dry surfaces compared to wet surfaces and Kusumaningrum et al. [17] also reported higher transfer of bacteria from wet sponges compared to dry sponges onto stainless steel coupons.
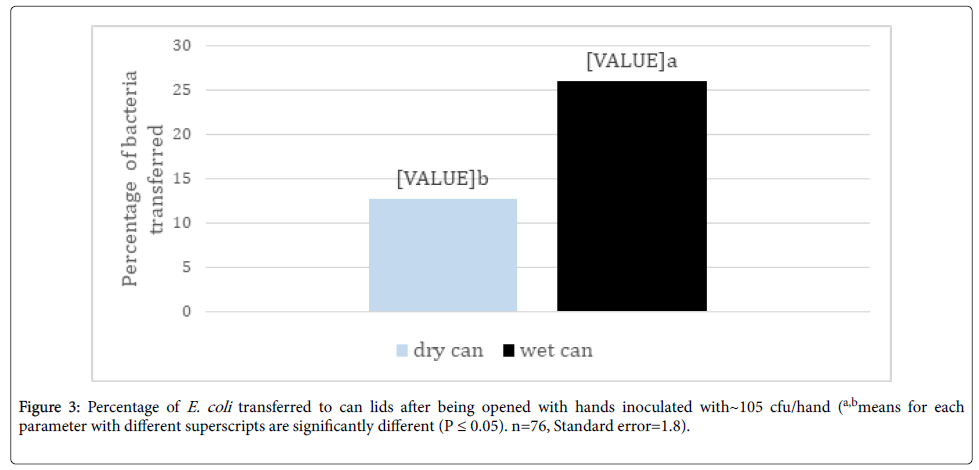
Figure 3. Percentage of E. coli transferred to can lids after being opened with hands inoculated with~105 cfu/hand (a,bmeans for each parameter with different superscripts are significantly different (P ≤ 0.05). n=76, Standard error=1.8).
Experiment 3
Effect of storage on survival of E. coli: When storage temperature treatments were pooled, there was a stepwise decrease in E. coli population with each increase in storage time (Figure 4). Also, there was greater survival of E. coli on cans held under refrigeration (4°C ± 2°C) after 1 day compared to cans held at room temperature (22°C ± 4°C) (Figure 4) , presumably due to the retention of moisture on the can lid surface under refrigeration compared to room temperature. However, by day 14, can lids at room temperature had more surviving cells that those held under refrigeration. More early as 1966, Hatch and Dimmick [18] reported that shifts in relative humidity (RH) had a greater effect on airborne bacterial survival than static RH levels, for example, Serratia marcescens died at a faster rate when the RH changed from 23% to 53% compared to being held at 53%. Lowering RH had similar effects on bacterial survival. Bacteria have shown that ability to survive and form biofilms on stainless steel surfaces. For instance, Kusumaningrum et al. [16] found that Staphylococcus aureus and Salmonella Enteriditis had ~4 and ~3 log populations surviving after 100 h on stainless steel from a 7 log initial population, respectively. Ryu and Beuchat [19] also found no decrease in E. coli O157:H7 biofilm population on stainless steel after 5 mins and only a reduction from 8 logs/coupon to 4 logs/coupon after 5 min treatment with 50 ug/ml chlorine. Staphylococcus aureus remained viable for at least 72 h on dry stainless steel surfaces and were capable of producing biofilms on wet surfaces after 24 h [20].
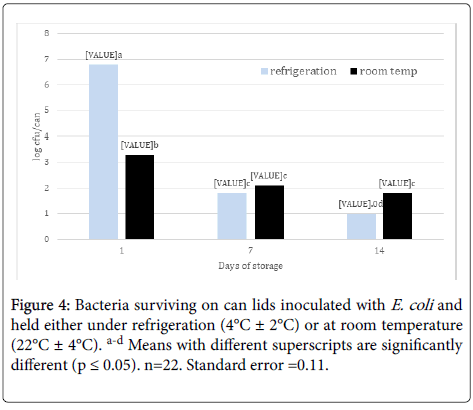
Figure 4. Bacteria surviving on can lids inoculated with E. coli and held either under refrigeration (4°C ± 2°C) or at room temperature (22°C ± 4°C). a-d Means with different superscripts are significantly different (p ≤ 0.05). n=22. Standard error =0.11.
The testing of randomly sampled can lids from home and markets with ATPase bioluminescence found that about 46% were in the “dangerously” unsanitary range with about 30% more in the “cautionary” unsanitary range. Also, opening a can with contaminated hands transferred 26 % of the bacteria to wet can and over 12% to dry can lids. Finally bacteria on inoculated can lids survived at least 14 days both under refrigeration and at room temperature. Some beverage can lids now have lid covers which would prevent contamination prior to opening. Future studies and environmental monitoring may include testing for likely pathogens on randomly sampled cans.
Citation: Dawson P, Aljeddawi W, Buyukyavuz A, Han I, Martinez-Dawson R (2018) Bacteria on Can Lids. J Food Microbiol Saf Hyg 3:140.
Received: 13-Nov-2018 Accepted: 02-Dec-2018 Published: 09-Dec-2018
Copyright: © 2018 Dawson P et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.