Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2022)Volume 13, Issue 6
Essential oils are highly concentrated substances extracted from flowers, leaves, stems, roots, seeds, barks, resins, or fruit rinds. These oils are often used for their flavor and their therapeutic or odoriferous properties, in a wide selection of products such as foods, medicines, and cosmetics. Extraction of essential oils is one of the most time and effort consuming processes. The way in which oils are extracted from plants is important because some processes use solvents that can destroy the therapeutic properties. There are wide number of ways to extract the essential oil but the quality never remains the same. The primary purpose of this study was to evaluate the various processes for extraction of essential oils from medicinal plant and screening that we use in our daily research. Extraction techniques include steam distillation, hydro distillation, cold pressing, maceration, super critical carbon dioxide, effleurage and dry distillation. Following that, the numerous methodologies outlined above might be grouped and addressed in terms of targeted biological testing to help young researchers focus and direct their research.
Extraction; Essential oil maceration; Hydro distillation
Essential oils, as an important compound, are oily liquids obtained from the stem, stock and leaf of plants, can be called as volatile, ethereal or essential oils. Essential oils, consisting of complicated mixtures of very low amounts volatile compounds, are present at different odor and aroma in several plants. Area of usage of essential oils is medicine, food, pharmacology and animal husbandry with the antioxidant and antimicrobial effects. The most prominent feature of these components, giving the herbaceous odor and taste, is to be volatile and fragrant at room temperature. Essential oils densely contain terpenoids, acids, alcohols, aldehydes, ketones, acyclic esters, lactones and rarely nitrogen and sulfur compounds, homologues of coumarins and phenylpropanoids [1].
Essential oils are used in a wide variety of consumer goods such as detergents, soaps, toilet products, cosmetics, pharmaceuticals, perfumes, confectionery food products, soft drinks, distilled alcoholic beverages (hard drinks) and insecticides. The world production and consumption of essential oils and perfumes are increasing very fast [2]. Essential oils are intense volatile aromatic compounds produced by plants the easily evaporated gist’s that give plants their wonderful scents. Each of these complex precious liquids is extracted from a particular species of plant life. Each plant species originates in certain regions of the world, with particular environmental conditions and neighboring fauna and flora. Essential oils are frequently referred to as the “life force” of plants. Unlike fatty oils, these "essential" oils are volatile, highly concentrated, substances extracted from flowers, leaves, stems, roots, seeds, bark, resin or fruit rinds. The amount of essential oils found in these plants can be anywhere from 0.01 percent to 10 percent of the total [3]. That's why tons of plant material is required for just a few hundred pounds of oil. These oils have potent antimicrobial factors, having wide range of therapeutic constituents. These oils are often used for their flavor and their therapeutic or odoriferous properties, in a wide selection of products such as foods, medicines, and cosmetics. Beware of imitations. Essential oils cannot be substituted with synthetics. Only pure oils contain a full spectrum of compounds that cheap imitations simply cannot duplicate [4].
Essential oils have vast applications for personal care, including beautifying the skin and hair, cleansing the mouth, gums, and teeth, and other uses for general hygiene. Because each essential oil has a unique chemical profile that provides different benefits, it is easy to use oils for a wide variety of tasks that relate to personal hygiene. Since ancient times, essential oils have been used to promote healthy skin, a clean mouth, strong fingernails and toenails, shiny hair, and more. Not only do natural cleansing, soothing, and purifying properties make essential oils an ideal choice for personal care, but the user can simultaneously enjoy the lovely, inviting smells of essential oils during use [5].
Methods of essential oil extraction
The choice of method of extracting essential oils depends on the initial condition and the characteristics of the raw material plant. The ratio of essential oils to raw material can vary widely from crop to plant and can range from 0.015% to over 20% [6]. The extraction method determines the characteristics of essential oils such as viscosity, color, melting, flexibility, and can cause enrichment or thinning of other components. Some of the extraction methods described below.
Steam distillation: It is a very popular method used to extract and separate essential oils from plants for use in natural products. This occurs when steam evaporates mutant plant elements, which eventually go through a process of summarizing and collecting [7]. The most important procedures to follow are these:
• A large container called a Still, which is usually made of stainless steel, containing the plant material has steam added to it.
• Through an inlet, steam is injected through the plant material containing the desired oils, releasing the plant’s aromatic molecules and turning them into vapor.
• The vaporized plant compounds travel to the condensation flask or the condenser. Here, two separate pipes make it possible for hot water to exit and for cold water to enter the condenser. This makes the vapor cool back into liquid form.
• The aromatic liquid by-product drops from the condenser and collects inside a receptacle underneath it, which is called a Separator. Because water and oil do not mix, the essential oil floats on top of the water. From here, it is siphoned off. (Some essential oils are heavier than water, such as clove essential oil, so they are found at the bottom of the separator) (Figure 1).
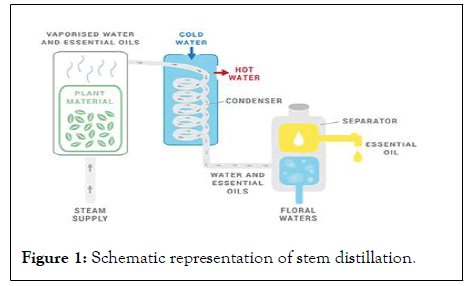
Figure 1: Schematic representation of stem distillation.
Distillation
Water distillation is used to extract essential oils from raw or dried plants by diffusion. The plants are soaked in the container, which has water to prevent overheating and charring of the plants, and then heated till the steam comes out. The oil comes out and it goes to the condenser, where the oil and water are collected. The oil collected in the top layer of hydrosol can be isolated. In this method, the extraction temperature is always below 100°C at the surface of the plants to avoid the evaporation of water and oil together. Heating systems in the extraction of essential oils using water distillation are direct fire, steam jacket, closed steam jacket, and closed or open steam coil [8] (Figure 2).
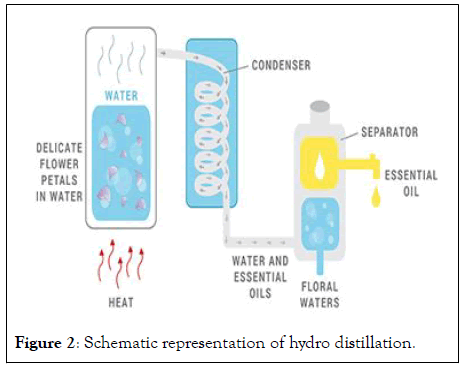
Figure 2: Schematic representation of hydro distillation.
Cold press extraction
These systems are used to collect oil in orange peels, for example, oranges, lemons, grapes and bergamots. These systems include basic skin compression at about 115 degrees for fat accumulation. These peels are separated from the natural product, concentrated and crushed or coated. Then outcomes are mixtures of Essentials oils, fluids which shall separate respective. A slight transformation occurs these citric oils carry their own fragrant, pure, uplifting scent similar to that of a highly organic product. This method is also called expression or scarification and is used for citrus pages in particular [9]. The main procedures are (Figure 3)
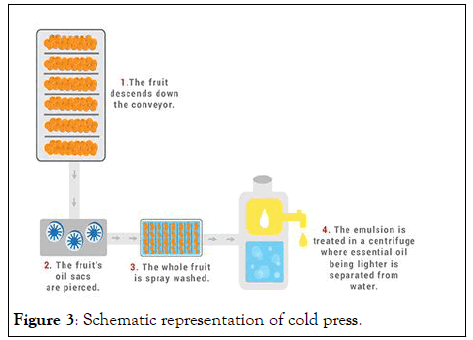
Figure 3: Schematic representation of cold press.
• Whole fruit is placed in a device that mechanically pierces it to rupture the essential oil sacs, which are located on the underside of the rind. The essential oil and pigments run down into the device’s collection area.
• Whole fruit is pressed to squeeze out the juice and the oil.
• Oil and juice that are produced still contain solids from the fruits, such as the peel, and must be centrifuged to filter the solids from the liquids.
• Oil separates from the juice layer and is siphoned off into another receptacle.
Solvent extraction
This method uses dietary solvents such as hexane and ethanol to separate essential oils from plant matter. It is best suited for plant products that produce low amounts of essential oils, most of which have frankincense, or soft aromatics that can withstand the pressure and stress of steam distillation. This method also produces a more pleasant aroma than any other method of digestion. Through this process, fixed plant materials, such as waxes and pigments, are also extracted and sometimes removed by other processes. When the plant is treated with solvent, it produces a fragrant compound called "concrete." When this concrete substance is mixed with alcohol, the oil particles are released. The chemicals mentioned above are used in this process and are then stored in oils and oils used in perfumes by the perfume industry or for aromatherapy purposes [10] (Figure 4).
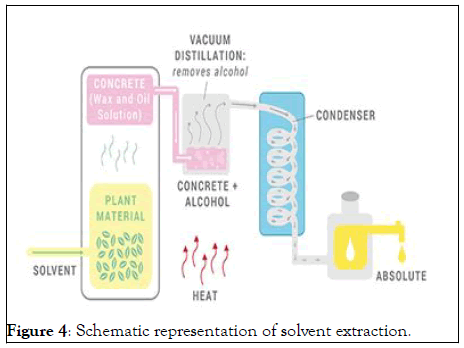
Figure 4: Schematic representation of solvent extraction.
Solvent extraction encompasses the following methods: Hypercritical CO2 (Carbon Dioxide), maceration, enfleurage.
Maceration: Maceration really makes a greater amount of "infused oil" as opposed to vital oils. Plants matters are absorbed vegetable soils, warmed or stressed and soon thereafter this might have utilized to back rub. These systems are alluring in light of the fact that this varies arrangement for oils. Maceration processes are in Figure 5.
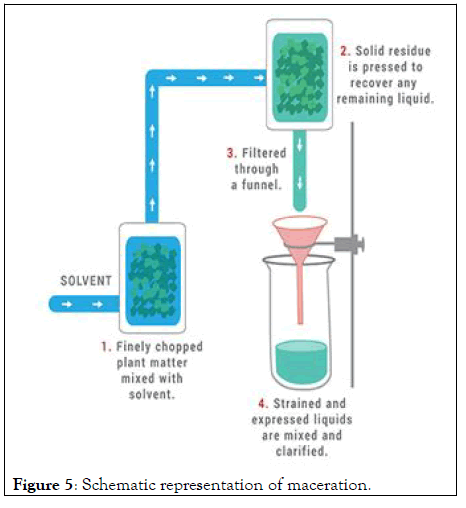
Figure 5: Schematic representation of maceration.
• Plant material is finely cut, crushed, or ground into moderately coarse powder.
• Plant material is placed in a closed vessel.
• Solvent (Menstruum) is added.
• The mixture is allowed to stand for 1 week and is shaken occasionally.
• The liquid is strained.
• Solid residue (Marc) is pressed to recover any remaining liquid.
• Strained and expressed liquids are mixed.
• Liquids are clarified through filtration or subsidence.
Super critical CO2 extraction: Supercritical CO2 extraction combines warm CO2 into 78 degree Ferrenhite under this condition; CO2 compared to heavy vapor with the arrival of weight in any transition, carbon dioxide escapes from its evaporating structure, leaving essential oils. The most common discharge system is steam cleaner. After extraction, the essential oil properties of decent quality should be as close as expected to the “representation” of the original plant. The way to the "essential oil" is to have a low weight and a low temperature conversion. High temperatures, rapid conversion and the use of solvents alter the structure of the underlying atom, will damage the usefulness and alter the aroma. CO2 emission processes are present (Figure 6).
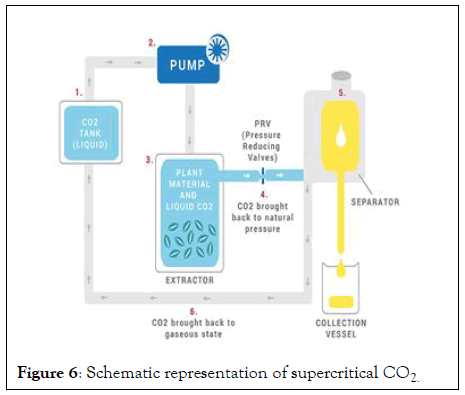
Figure 6: Schematic representation of supercritical CO2.
• Pressurized carbon dioxide becomes liquid while remaining in a gaseous state, which means it, is now "supercritical." In this state, it is pumped into a chamber filled with plant matter.
• Because of the liquid properties of the gas, the CO functions as a solvent on the natural plant matter, pulling the oils and other substances such as pigment and resin from the plant matter. The essential oil content then dissolves into the liquid CO.
• The CO is brought back to natural pressure and evaporates back into its gaseous state, while what is left is the resulting oil.
CO2 is colorless, odorless, and can be easily and completely removed by releasing the pressure in the extraction chamber. It is what we exhale and is needed by plants in order for them to thrive, which illustrates its harmlessness when employed in the extraction process. This absence of potentially harmful solvents in CO2 extraction means neither the human body nor the environment is polluted (Figure 6).
Enfleurage: Enfleurage is rarely used today, but it is one of the oldest extracts from essential oils. At the end of the process, vegetable oil or animal fats are added to the fragrance of flowers. The oil used is odorless and solid at room temperature. The enfleurage process can be done "hot" or "cold." In both cases, the fat that is saturated with fragrance is called "enfleurage pomade." The only difference between cold and hot effleurage is that the oil heats up. Procedures for effleurages are
• Highly purified and odorless vegetable or animal fat, usually lard or tallow, is spread out over glass plates in a frame called a chassis and is allowed to set.
• Fresh flower petals or fresh whole flowers are then placed on top of the layer of fat and pressed in. They are allowed to set for 1-3 days or for a couple of weeks depending on the flowers that are used. During this time, their scent seeps into the fat.
• The depleted petals are replaced and the process is repeated until the fat reaches the desired saturation.
• The final product is the enfleurage pomade: the fat and the fragrant oil. This is washed with alcohol to separate the botanical extract from the remaining fat, which is used to make soap. When the alcohol evaporates from this mixture, the “absolute” is what is left over. The only difference between cold and hot enfleurage is that the fats are heated (Figure 7).
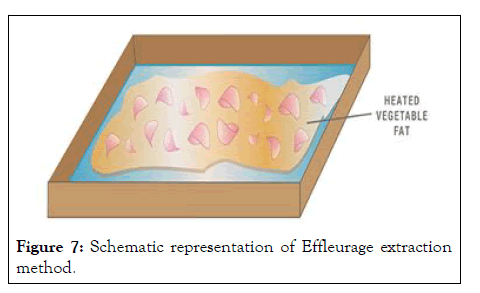
Figure 7: Schematic representation of Effleurage extraction method.
Dry distillation
The essential oil is produced by high temperature heating of stems or barks in a suitable apparatus without the addition of water or steam. It is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The product is condensed and collected (Figure 7).
Essential oils are biologically important substances produced by aromatic plants that are stored in specialized glands, roots and leaf hairs. Among their many functions they can act as insect repellants and pollinator attractants or serve as antimicrobial agents when under attack but to most humans, they simply smell good. Essential oils may be released from the plant and collected (concentrated) under the conditions of high-pressure steam distillation, hydro distillation, dry distillation, cold pressing and solvent extraction, or a combination thereof. Although these methods are predominantly exploited on the laboratory scale, they have also found industrial applications, although in most cases to a limited extent.
Citation: Asfaw MD (2022) Basic Essential Oil Extraction Techniques and Procedures from Aromatic Plants. J Chromatogr Sep Tech. 13:489.
Received: 02-Jun-2022, Manuscript No. JCGST-22-17715; Editor assigned: 06-Jun-2022, Pre QC No. JCGST-22-17715 (PQ); Reviewed: 22-Jun-2022, QC No. JCGST-22-17715; Revised: 30-Jun-2022, Manuscript No. JCGST-22-17715 (R); Published: 08-Jul-2022 , DOI: 10.35248/2157-7064.22.13.489
Copyright: © 2022 Asfaw MD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.