Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Editorial - (2014) Volume 5, Issue 2
Nuclear magnetic resonance, NMR, is a modern spectroscopic technique which has been applied in a diversity of disciplines including chemistry, biochemistry, materials science and geoscience. NMR spectroscopy is primarily concerned with interactions between isolated spin pairs. The NMR Hamiltonian operator for an isolated spin pair in an applied external magnetic field, B0, is given by
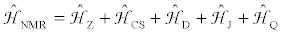 (1)
(1)
where 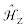 represents the Zeeman interaction and
represents the Zeeman interaction and 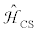 represents the nuclear magnetic shielding interaction;
represents the nuclear magnetic shielding interaction; 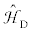 and re
and re present the direct dipolar and indirect nuclear spin-spin coupling interactions, respectively. The last term
present the direct dipolar and indirect nuclear spin-spin coupling interactions, respectively. The last term 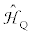 denotes the quadrupolar interaction for quadrupolar nuclei.
denotes the quadrupolar interaction for quadrupolar nuclei.
All isotopes in periodic table are NMR active nuclei except those with even atomic numbers and even mass numbers (e.g., 12C, 16O, etc.). Isotopes with a nuclear spin quantum number, I=½, are the most commonly observed nuclei in NMR experiments, including 1H, 1C, 15N, 19F, 29Si, 31P, 107,109Ag, 195Pt and 203,205Tl [1]. Quadrupolar nuclei are those with spin (I) greater than 1/2, and constitute ca. 70% of NMR active nuclei in the Periodic Table as indicated in Figure 1, such as 2D (I=1), 7Li (I=3/2), 11B (I=3/2), 17O (I=5/2), 23Na (I=3/2), 27Al (I=5/2), 69,71Ga (I=3/2) and 113,115In (I=9/2). From the analysis of the NMR spectrum, useful information such as structural and dynamic information could be determined. The information, arising from different NMR interactions shown in Eq. (1), includes the chemical shift, CS, tensor and the indirect spin-spin coupling constants, J, between coupled nuclei. For quadrupolar nuclei, one also can extract the electric field gradient, EFG, tensors for these nuclei.
Depending on the states of the matter studied, NMR spectroscopy can be mainly classified into two categories: solution and solid-state NMR (SSNMR). Solution NMR generally studies compounds dissolved in low-viscosity solvents and high-resolution NMR spectra can be obtained, which is the main advantage of this technique over SSNMR. The high-resolution NMR spectra in solution arise from the averaging of several NMR interactions due to rapid molecular tumbling. In contrast, the absence of rapid molecular motion for solid samples leads to broad peaks in SSNMR spectra that often contain spectral features. However, the development of the magic-angle spinning (MAS) technique allows the spectroscopists to produce “solution-like” NMR spectra of solid samples, from which similar information as from solution NMR can be obtained. Figure 2 shows an example of solidstate 109Ag NMR spectra of AgSO3CH3 acquired with cross-polarization (CP) enhancement technique under MAS or stationary conditions [2]. From the analysis of the features in the solid-state 109Ag NMR spectra of MAS and stationary samples, CS tensor including isotropic (δiso) and anisotropic (δ11, δ22 and δ33) chemical shift values can be determined. For quadrupolar nuclei, additional information such as EFG tensor and the relative orientation of CS and EFG tensors can also be determined. There are also several other advantages for SSNMR spectroscopy; for example, it is available to insoluble samples. In addition, many compounds do not maintain their solid-state structure or are unstable when dissolved in a solvent. Thus SSNMR spectroscopy can provide structural and dynamic information unavailable from solution NMR and other techniques such as single-crystal X-ray diffraction (XRD) due to the difficulties to grow suitable single crystals for examination by XRD.
Figure 2: (a) 109Ag CP/MAS NMR spectra of AgSO3CH3 at different spinning rates, νrot = 830 Hz (lower trace) and at νrot = 3 kHz (upper trace); the asterisk refers to δiso and the spikes refer to spinning sidebands. (b) 109Ag NMR spectrum of a stationary sample of AgSO3CH3. (a-b) B0 = 11.75 T [2]. The shift values are referenced to AgN03 (1M) (δ(109Ag) = 0 ppm).
In the field of heterogeneous catalysis, SSNMR spectroscopy is a very useful tool for the investigation of framework atoms and extraframework species including surface sites of solid catalysts, reactants, adsorbate complexes and reaction intermediates formed on these materials. For extra-framework species, many nuclei such as 1H, 2D, 13C, 15N, 19F and 31P are NMR active which could be used to study the Brönsted and Lewis acid/base sites of solid catalysts, dynamic information, reaction intermediates and even in/ex situ reactions on these catalysts. Herein, we will not discuss these studies in detail and many reviews and textbooks have been published such as refs [3-11] and references therein.
Tetrahedral SiO4 units in the framework of zeolite catalysts are the basic structural blocks and various metal atoms including Al, B, Ga and Ti can be incorporated to these SiO4 units. In the case of Al atoms incorporated with SiO4 units, there are up to five different environments surrounding the tetrahedrally coordinated silicon atoms (Q4), denoted Si(nAl) with n=0, 1, 2, 3 and 4 [10]. For each type of Si(nAl) species, a 29Si MAS NMR signal is shown in a well-defined range of chemical shifts, as shown in Figure 3. In addition, the nSi/ nAl ratio in the framework of zeolite catalysts can be calculated via Eq. (2) [10]. Similarly, 29Si MAS NMR spectra can provide the framework chemical compositions for the gallium [11] and zinc [12] analogues of zeolite catalysts. Also, useful structural information of zeolite catalysts can be acquired from solid-state 17O and 27Al NMR spectra [8].
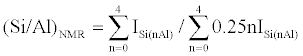 (2)
(2)
As briefly described above, SSNMR spectroscopy is a very useful technique for the investigation of local structure, dynamics and reactions on solid catalysts. Nowadays, SSNMR spectroscopy has become a routine method for the characterization of solid catalysts in recent decades due to the development of new techniques and the increase of commercial magnetic field strength. Thus, it would be expected that SSNMR will play more and more important roles for the research in the field of heterogeneous catalysis.