Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Commentary - (2022)
Benzoic Acid (BA) and some of its derivatives are naturally present in microbes, plants, and mammalian cells where they serve as precursors for generating many important metabolites such as hormones, enzyme cofactors, and defense molecules [1]. BA also has some protective effects, against bovine enterovirus type I for example, which is why the inclusion of BA in veterinary hygiene products has been recommended by the regulation of European Union [2]. External doses of BA in the form of sodium benzoate are used in humans to treat hyperammonemic condition that arises from genetic defect in the urea cycle [3]. Against these seemingly beneficial effects are detriments. For example, BA leads to allergic reactions, in asthmatics in particular [4]. Sodium benzoate used in the therapeutic regimen is thought to produce neurotoxic effect, damage liver and kidney [5], produce fetal abnormalities [6], and inhibit the respective activities of the enzymes trypsin and chymotrypsin even when administered at concentrations lower than the recommended limit [7]. These mentions raise the possibility that there could be more unknown attributes of BA, compounding the controversies surrounding.
Whether conscious of these controversies or not, benzoates for a long time have been used extensively in foods and beverages, pharmaceuticals, and health and beauty products as approved additives. The Benzoate Class (BC) of food preservatives includes Benzoic Acid (BA), Methyl-Para Hydroxy Benzoic Acid (MPBHA), and Propyl-Para Hydroxy Benzoic Acid (PPBHA), although the last one is presently withdrawn perhaps due to its effect on steroidogenesis. These additives are identified as BA (210), MPHBA (218), and PPHBA (216) where the numbers within braces indicate International Numbering System (INS) as recommended by Codex Alimentarius Commission [8]. BA is a prescribed antimicrobial and flavoring agent in foods and beverages, MPBHA is an approved additive in processed foods and medications [9], and PPHBA is a recommended antifungal and flavoring agent in foods and pharmaceuticals [8,9]. The additive BA is used as it is when foods and beverages are to be preserved under acidic conditions (pH 2.5-pH 4), and MPHBA and PPHBA are used as esterified derivatives, commercially called methyl and propylparabens, respectively, for preservation in the 3-8 range of pH. Often, BA is added in conjunction with parabens for low-pH condition of preservation.
Consider the controversies surrounding benzoates, and pose a question concerning the amount of preservatives foods carry. There are of course guidelines and recommendations issued by regulatory bodies as to how much of preservatives could be added to foods. For example, 0.2% BA at pH 5 is the minimum concentration to prevent the growth of the common mold Aspergillus niger in food. The growth of the same mold can be inhibited by 0.125 (± 0.025)% methylparaben or 0.04 (± 0.01)% propylparaben across the pH range 5-9. In fact, the Food and Drug Administration (FDA) of the US health services, and the joint expert committee of Food and Agriculture Organization (FAO) and World Health Organization (WHO) have provided ‘Generally Regarded as Safe’ (GRAS) values of additives. For example, the use of methylparaben up to a level of 0.1% is considered safe.
Now there are two issues to work on. One, manufacturers are expected to adhere to good manufacturing practices by following the guidelines, not to exceed the limit of the preservatives set by the regulatory bodies. Often though, manufacturers do not declare the level of benzoates and paraben contents in foods. Even if declared, the veracity of the claim is cumbersome to check. Two, is there any scope to minimize the recommended level already set by the regulatory bodies? Putting another way, were sufficient checks and evaluations carried out to lay down the safe levels of the preservatives? Although the assessment documents of the regulatory agencies do cite evidences to arrive at the GRAS levels of the preservatives, finer information of experimental results are not included because either the existing results are not publicized or sufficiently detailed testing have not been carried out.
To highlight the importance and the outcome of the second point above, that is basic experimentation on the use of benzoate food preservatives, we refer to a few interesting results from a paper published in the December 2021 issue of ACS Food Science and Technology [10]. The effect of different concentrations of BC food preservatives on the conformations of three globular proteins- myoglobin, lysozyme, and cytochrome c, have been analyzed in the 4-7 pH range in which benzoates
BA, MPHBA, and PPHBA) are used as commercial preservatives. Nuclear Magnetic Resonance (NMR), Infrared (IR), and fluorescence anisotropy measurements show that all three benzoates bind to proteins by hydrogen bonding and aromatic -stacking interactions. The benzoate-bound proteins exhibit substantial drift from their native-state conformations, and enter the aggregation pathway within hours of benzoate addition. Electron micrographs of the benzoate-bound proteins show amorphous, and occasionally fibrillar, aggregates. For example, (Figure 1) shows a more recently acquired electron micrograph of an Intrinsically Disordered Protein (IDP) bound to 0.03 mM BA at pH 5. Notably, the BC food preservatives can induce protein aggregation even when used at concentrations much lower than the GRAS levels mentioned in the recommendation documents of regulatory bodies and FAO of the United Nations. The illustrated aggregate-inducing concentration of BA (0.03 mM) compares with 0.2% (~16.4 mM) thought to be required to inhibit aspergillus growth, and 50-1000 mg L -1 (~0.4-8.2 mM) recommended by FAO-WHO [11].
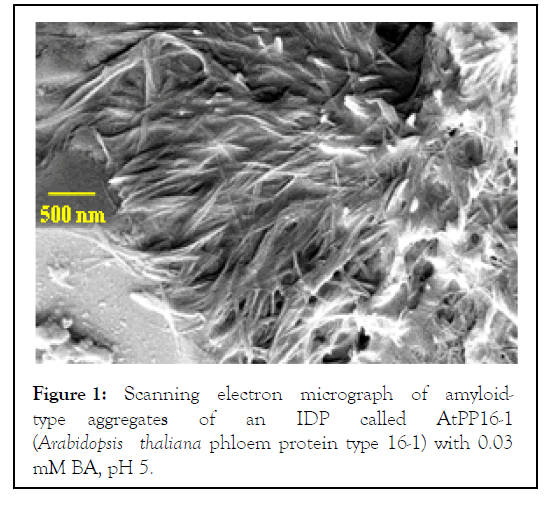
Figure 1: Scanning electron micrograph of amyloidtype aggregates of an IDP called AtPP16-1 (Arabidopsis thaliana phloem protein type 16-1) with 0.03 mM BA, pH 5.
We have no prejudice in the projection of abusive levels of BC food additives in use, but we offer an advisory here. There must be a collective effort to minimize the content of BC preservatives until their substitutes are found. Minimizing benzoates in foods must also be considered with regard to the effect of BA on liver. Generally benzoate is removed from liver by BA-glycine conjugation in the form of hippuric acid, but prolonged load of BA in the liver due to excess BC additives in foods may lead to liver damage [5].
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Bhuyan KA, Ramesh H (2022) Benzoate Uses as Food Preservatives. Food Microbial Saf Hyg. S3:002.
Received: 01-Feb-2022, Manuscript No. JFMSH-22-16418; Editor assigned: 04-Feb-2022, Pre QC No. JFMSH-22-16418 (PQ); Reviewed: 18-Feb-2022, QC No. JFMSH-22-16418; Revised: 24-Feb-2022, Manuscript No. JFMSH-22-16418 (R); Published: 03-Mar-2022 , DOI: 10.35248/2476-2059-22.S3.002
Copyright: © 2022 Bhuyan KA, et al. This is an open-accessarticle distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.