
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2018) Volume 7, Issue 4
There have been many reports that osmotic fragility (OF) in red blood cells (RBCs) is a valuable tool for assessing the actions of various chemicals on the cell membrane in vitro. We determined the effects of benzoic acid and its derivatives on OF in sheep RBCs in vitro. Isolated sheep RBCs were exposed to these substances at 0-100 mM in a buffer solution for 1 h, and the 50% hemolysis was then determined by soaking in 0.1-0.8% NaCl solution. OF was determined as the NaCl concentration inducing 50% hemolysis which was colorimetrically measured by the released hemoglobin concentration. Benzoic acid decreased OF in a dose-dependent manner. Heptanoic acid and cyclohexane carboxylic acid, both of which have 6 carbons in their saturated hydrocarbon structure, did not change OF. Replacement of the COOH bound to the benzene ring with PO(OH)2 or SO2OH abolished the OF response obtained by benzoic acid. Replacement with OH did not affect OF up to 25 mM, but did induce hemolysis at 50 and 100 mM. Replacement with CONH2 decreased OF, with the degree of the OF-lowering effect being greater than that of benzoic acid. Most derivatives possessing other groups (OH, CH3 or NH3) or halogens (Cl or Br) decreased or tended to decreased OF, with the degree of change in OF dependent on the position of the group introduced onto the benzene ring. 4-methylbenzoic acid, and 3-,4- and 4-, 5-dichlorobenzoic acids demonstrated a biphasic effect on OF in sheep RBCs; lowering OF up to 50 mM followed by a lytic effect at 100 mM. The results of a regression analysis using the values of all substances tested revealed no significant correlations between the partition coefficient of the substances and their effects on OF response. However, some groups of substances at concentrations of 10-50 mM showed a negative and statistically significant correlation. For substances sharing very close chemical structures, partition coefficients can probably be used as an indicator for evaluating the effect on OF within an appropriate range of concentrations.
Keywords: Benzoic acid; Partition coefficient; Osmotic fragility; Erythrocyte; Membrane; Sheep
Benzoic acid is a monocarboxylic acid composed of a hydrophilic carboxylic base and a hydrophobic benzene nucleus, and is classified as an aromatic hydrocarbon. Benzoic acid and its derivatives naturally occur as components in plants and mushrooms [1,2] and their metabolic route in these natural resources have been shown in detail [3,4]. Many types of esters in benzoic acid and its analogues are used as artificial aromas in cosmetics and processed food [5,6]. Benzoic acid and its sodium salts are widely added to food as preservatives or antifungal drugs [5,6]. Various biological effects have been shown for many other chemical derivatives of benzoic acid such as acetylsalicylic acid, which is commonly used as an anti-febrile and painkilling drug [7]. In addition to these well-known physiological and pharmacological actions, benzoic acid and its derivatives have also been reported to have various biological actions both in vivo [8,9] and in vitro [8,10,11].
It was speculated that benzoic acid and its derivatives probably affect the cell membrane directly, thereby inducing biological actions in various tissues, particularly as described in the in vitro experiments mentioned above. As red blood cells (RBCs) are composed of a biological membrane and lack a nucleus and intracellular organelle, RBCs are used as a prototypical cellular model for examining chemical-mediated effects on the plasma membrane. Moreover, their hemolytic response following changes in the membrane is easily determined by spectrophotometry through the release of hemoglobin. Previous studies in our laboratory have shown that osmotic fragility (OF), determined as the 50% hemolysis of RBCs in rats, is a useful indicator for evaluating interactions between monocarboxylic acids and the cell membrane in vitro [12,13]. The effects of benzoic acid, a monocarboxylic acid, and its chemical derivatives on the cell membrane have also been evaluated by the measurement of OF in rat RBCs in vitro [14]. As pre-treatment of the rat RBCs with trypsin did not affect the increase in OF induced by some monocarboxylic acids [12] and benzoic acid [14], these carboxylic acids are thought to directly affect and change the characteristics of the RBC membrane in vitro.
As various types of benzoic acid-related substances are available, these chemical analogues afford a useful set of tools for the detailed examination of the structure-activity relationships of OF in RBCs. Our series of experiments have shown that benzoic acid and its analogues induced OF responses in a chemical structure-depend manner in rat [14] and guinea pig RBCs [15]. Exposure to benzoic acid and some of its derivatives increase OF in rat RBCs in vitro [14]. Contrary to this observation, the same experimental procedures revealed that benzoic acid and most of its derivatives decreased OF in guinea pig RBCs [15]. For monocarboxylic acids possessing straight hydrocarbon chains, we have shown that some of these acids increased OF in rat RBCs [15,16] and decreased OF in guinea pig RBCs [16]. It was speculated that the different effects of monocarboxylic acids on OF between rat and guinea pig RBCs were due to differences in RBC membrane characteristics, particularly with regard to the phospholipid composition [16].
Benzoic acid and its related substances are speculated to enter the membrane and interact with the phospholipid layers, thereby inducing changes in OF in RBCs. The permeation of these chemicals into the RBC membrane is thought to be an important factor in inducing changes in membrane resistance to osmotic pressure. The partition coefficient of a chemical substance is one of the physicochemical values which indicate the potential for the permeation or delivery of chemicals into the membrane [17-20]. The octanol/water permeation coefficients have been widely used as an indicator of the distribution of chemicals in cells, tissues and the body [21-23]. The values for various substances are commonly provided on many websites.
The objective of this experiment was to examine the effect of benzoic acid and its related substances on OF, and clarify the relationship between the partition coefficient of the tested substances and their effects on OF in sheep RBCs. These data can provide us with clues to understanding the structure-activity relationships between benzoic acid-related substances and their potential to affect the RBC membrane. In addition, we compared the OF response to benzoic acid and its analogues between the sheep RBCs in the present study and the rat and guinea pig RBCs previously reported by our laboratory. This comparison affords us with a new understanding of the relationship between the OF response to carboxylic acids and the composition of the RBC membrane in various mammal species.
Reagents
Biochemical grade benzoic acid, heptanoic acid, cyclohexanecarboxylic acid, benzenephosphonic acid, benzenesulfonic acid, benzamide, hydroxybenzene (phenol), 2-, 3-, and 4-hydroxybenzoic acid, 2-, 3-, and 4-methylbenzoic acid, 2-, 3-, and 4-aminobenzoic acid, 2-, 3-,4-chlorobenzoic acid, 2-, 3-, and 4-bromobenzoic acid, 2,3-, 2,4- , 2,5-, 2,6-, 3,4-, and 3,5-dihydroxylbenzoic acid, and 2,3-, 2,4-, 2,5-, 2,6-, 3,4-, and 3,5-dichlorobenzoic acid were purchased from Tokyo Kasei Kogyo Co., Ltd (Tokyo, Japan) or Wako Pure Chemical Co., Ltd. (Osaka, Japan). The chemical structures of these substances are shown in tables in the Results section. All other reagents used in this study were analytical grade.
Preparation of sheep RBCs
Sheep blood was purchased from the Ecorin Village Farm (Eniwa, Hokkaido). Mixed breed female sheep aged 3 to 5 years old (n=20, body weight 70-85 kg) were used for blood sampling. Animals were given free access to a pelleted diet, hay and tap water. Each blood sample (30 ml) was collected from the left jugular vein into a heparinized test tube through a hypodermic needle by a veterinarian. The blood was immediately transported to our laboratory and kept in a refrigerator at 4ºC for about 18 hours. RBCs were separated from the plasma by centrifugation at 2000 g for 15 min (Model 2420, Kubota Inc., Tokyo, Japan). The plasma and buffy coat were removed by aspiration. The crude RBCs obtained were then washed three times with two volumes of cold 0.9% NaCl solution. A dense-packed cell suspension was obtained and thereafter kept in ice-cold water until subsequent treatment.
Experimental procedure
The experimental procedures followed to those in our previous report [14]. The packed RBC suspension prepared above (30 μl) was transferred into 0.6 ml of phosphate-NaCl buffer solution (PH 7.4) containing benzoic acids or its derivatives at 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50 and 100 mM in 1.5-ml micro test tubes (Nichiryo Co., Ltd., Tokyo, Japan). The appropriate amount of NaCl was added to the buffer solution to adjust the osmolarity when each chemical was tested. When the effect of the benzoic acid (parent chemical) and its derivatives, or intra-group substances was compared, RBCs obtained from the same sheep were used for determining OF. The suspensions of RBCs applied to chemical substances were incubated by shaking (1 stroke/sec) at 37ºC for 1 hr (Shaking Bath TBK 202 DA, Advantec Co., Ltd., Tokyo, Japan). Each RBC suspension was gently mixed by a mixer (Vortex Genie 2, model-G560, Scientific Industry, Inc. NY., USA) following incubation, and 50 μl of each suspension was transferred into a 96-deep-well microplate (2 ml volume, Whatman Inc., Piscataway, NJ, USA) containing a 1 ml NaCl solution ranging from 0.1 to 0.8%. The deep-well microplate was immediately centrifuged at 1300 g (Plate Spin II, Kubota Inc., Tokyo, Japan) for 10 min at room temperature. A 200 μl portion of the supernatants containing various concentrations of hemoglobin derived from the burst RBCs was transferred into another 96-well microplate with a volume of 300 μl (Whatman Inc., Piscataway, NJ, USA) and were determined colorimetrically at 540 nm (Microplate Reader Model 680, Bio-Rad Laboratories, Tokyo, Japan).
Statistical analysis
Complete hemolysis of the RBC suspension occurred in the 0.1% NaCl solution, for which the hemoglobin concentration was defined as 100%. Hemolysis of the RBCs did not occur in the 0.8 % NaCl solution, for which the hemoglobin concentration was defined as 0%. The effective concentration of the NaCl solution inducing 50% hemolysis (EC50) of the applied RBCs was calculated from the hemolysis curve by using a straight-line equation between the points immediately adjacent to 50%. OF in the RBCs was expressed as the EC50 value (NaCl %). All values are expressed as means ± S.D. (n=6). Dunnett’s test following one-way ANOVA was used to determine the significance of the differences between the control (0 mM) and subsequent concentrations (0.1-100 mM). As obvious changes in OF were obtained by the application of most carboxylic acids at 10, 25, 50 and 100 mM, the differences from the control value at 0 mM were calculated and expressed as ΔEC50 (NaCl %). The partition coefficients of the substances examined in this experiment were quoted from the PubChem [24], the ChemSpinder [25] or the ChemIDpuls [26] websites for chemical and physical properties. Regression analysis was used to confirm the relationship between the partition coefficient of each carboxylic acid and the ΔEC50 of the RBCs. Statistical analyses were performed using Excel Tokei for Windows 2012 (SSRI Co., Ltd., Tokyo, Japan). Statistical significance was set at a P value < 0.05 or 0.01.
Representative OF responses induced by the tested substances at the concentrations from 0 (control) to 100 mM are shown in Figures 1-5. The partition coefficient and ΔEC50 at concentration of 10, 25, 50 and 100 mM of all tested substances are summarized in Tables 1-5.
Figure 1: Effects of benzoic acid and its related substances on OF in sheep RBCs. (A): The effects of benzoic acid (●) and heptanoic acid (▲), and (B): benzenephosphonic acid (●) and benzamide (▲) are presented. Values are the means ± SD (n=6). Open symbols indicate that there was a significant difference between the control (0 mM) and subsequent concentrations (0.1- 100 mM) based of Dunnett’s test (P<0.05, including the case of P<0.01 in Table 1).
Figure 2: Effects of benzoic acid derivatives in which H on the benzene ring was replaced with OH or CH3 on OF in sheep RBCs. (A): The effects of 2-hydroxybenzoic acid (●) and 4-hydroxybenzoic acid (▲), and (B): 2-methylbenzoic acid (●) and 4-methylbenzoic acid (▲) acid are presented. Values are the means ± SD (n=6). Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100 mM) based of Dunnett’s test (P<0.05, including the case of P<0.01 in Table 2).
Figure 3: Effects of benzoic acid derivatives in which H on the benzene ring was replaced with Cl or Br on OF in sheep RBCs. (A): The effects of 2-chlorobenzoic acid (●and 4-chlorobenzoic acid (▲), and (B): 2-bromobenzoic acid (●) and 4-bromobenzoic acid (▲) acid are presented. Values are the means ± SD (n=6). Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100 mM) based of Dunnett’s test (P<0.05, including the case of P<0.01 in Table 3).
Figure 4: Effects of benzoic acid derivatives in which H at two positions on the benzene ring was replaced with two OH groups on OF in sheep RBCs. (A): The effects of 2,3-hydroxybenzoic acid (●) and 2,4-hydroxybenzoic acid (▲), and (B): 2,6-hydroxybenzoic acid (●) and 3,4-hydroxybenzoic acid (▲) acid are presented. Values are the means ± SD (n=6). Open symbols indicate that there was a significant difference between the control (0mM) and subsequent concentrations (0.1-100 mM) based of Dunnett’s test (P<0.05, including the case of P<0.01 in Table 4).
Figure 5: Effects of benzoic acid derivatives in which H at two positons on the benzene ring was replaced with two Cl atoms on OF in sheep RBCs. (A): The effects of 2,3-chlorobenzoic acid (●) and 2,4- chlorobenzoic acid (▲), and (B): 2,6- chlorobenzoic acid (●) and 3,4- chlorobenzoic acid (▲) acid are presented. Values are the means ± SD (n = 6). Open symbols indicate that there was a significant difference between the control (0 mM) and subsequent concentrations (0.1-100 mM) based of Dunnett’s test (P<0.05, including the case of P<0.01 in Table 4).
| Substance | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|
| Benzoic acid | 1.87 | 10 | 0.001 ± 0.012 | |
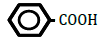 |
25 | -0.014 ± 0.015 | ||
| 50 | -0.038 ± 0.022 | ** | ||
| 100 | -0.076 ± 0.031 | ** | ||
| Hepanoic acid | 2.42 | 10 | 0.003 ± 0.015 | |
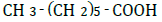 |
25 | 0.002 ± 0.010 | ||
| 50 | 0.000 ± 0.008 | |||
| 100 | -0.018 ± 0.033 | |||
| Cyclohexanecarboxylic acid | 1.96 | 10 | 0.007 ± 0.010 | |
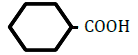 |
25 | 0.000 ± 0.016 | ||
| 50 | -0.012 ± 0.021 | |||
| 100 | -0.018 ± 0.035 | |||
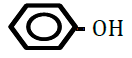
| Hhydroxybenzene (phenol) | 1.46 | 10 | 0.015 ± 0.012 | |
 |
25 | 0.033 ± 0.019 | ||
| 50 | No data (burst) | |||
| 100 | No data (burst) | |||
| Benzenephosphonic acid | 0.54 | 10 | 0.016 ± 0.010 | |
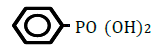 |
25 | 0.016 ± 0.011 | ||
| 50 | -0.002 ± 0.028 | |||
| 100 | -0.024 ± 0.022 | |||
| Benzenesulfonic acid | -1.2 | 10 | -0.006 ± 0.012 | |
 |
25 | -0.013 ± 0.015 | ||
| 50 | -0.011 ± 0.008 | |||
| 100 | -0.006 ± 0.024 | |||
| Benzamide | 0.64 | 10 | -0.059 ± 0.017 | * |
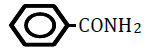 |
25 | -0.061 ± 0.012 | * | |
| 50 | -0.066 ± 0.012 | * | ||
| 100 | -0.087 ± 0.019 | ** | ||
Table 1: Effects of benzoic acid and its related substances in which the benzene ring or a carboxylic group was replaced with another element on OF in sheep RBCs. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [21] website. The ΔEC50 values (amount of change in NaCl %) at 10, 25, 50 and 100 mM are presented. Asterisks (* and **) indicate that there was a significant difference (P<0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test.
| Substance | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|
| 2-Hydroxybenzoic acid | 2.26 | 10 | -0.024 ± 0.009 | |
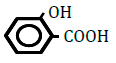 |
25 | -0.048 ± 0.015 | * | |
| 50 | -0.081 ± 0.016 | ** | ||
| 100 | -0.080 ± 0.018 | ** | ||
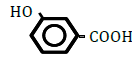
| 3-Hydroxybenzoic acid | 1.50 | 10 | -0.001 ± 0.018 | |
 |
25 | -0.015 ± 0.017 | ||
| 50 | -0.036 ± 0.016 | |||
| 100 | -0.065 ± 0.033 | * | ||
| 4-Hydroxybenzoic acid | 1.58 | 10 | -0.013 ± 0.011 | |
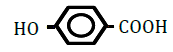 |
25 | -0.024 ± 0.014 | ||
| 50 | -0.035 ± 0.005 | |||
| 100 | -0.062 ± 0.019 | * | ||
| 2-Methylbenzoic acid | 2.46 | 10 | -0.005 ± 0.020 | |
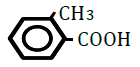 |
25 | -0.007 ± 0.013 | * | |
| 50 | -0.022 ± 0.028 | ** | ||
| 100 | -0.030 ± 0.021 | ** | ||
| 3-Methylbenzoic acid | 2.08 | 10 | -0.011 ± 0.021 | |
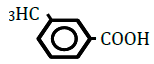 |
25 | -0.042 ± 0.018 | * | |
| 50 | -0.068 ± 0.030 | ** | ||
| 100 | -0.085 ± 0.036 | ** | ||
| 4-Methylbenzoic acid | 2.27 | 10 | -0.020 ± 0.008 | |
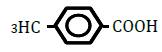 |
25 | -0.066 ± 0.046 | * | |
| 50 | -0.148 ± 0.030 | ** | ||
| 100 | No data (burst) | |||
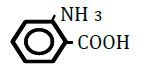
| 2-Aminobenzoic acid | 1.21 | 10 | -0.014 ± 0.007 | |
 |
25 | -0.022 ± 0.013 | ||
| 50 | -0.051 ± 0.014 | ** | ||
| 100 | -0.081 ± 0.022 | ** | ||
| 3-Aminobenzoic acid | 0.65 | 10 | -0.004 ± 0.022 | |
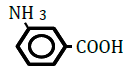 |
25 | -0.016 ± 0.019 | ||
| 50 | -0.026 ± 0.028 | |||
| 100 | -0.031 ± 0.021 | |||
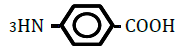
| 4-Aminobenzoic acid | 0.83 | 10 | -0.003 ± 0.003 | |
 |
25 | -0.014 ± 0.018 | ||
| 50 | -0.037 ± 0.013 | |||
| 100 | -0.062 ± 0.021 | ** | ||
Table 2: Effects of benzoic acid derivatives in which hydrogen was replaced with a hydroxy, methyl or amino group on the benzene ring on OF in sheep RBCs. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [21] website. The ΔEC50 values (amount of change in NaCl %) at 10, 25, 50 and 100 mM are presented. Asterisks (* and **) indicate that there was a significant difference (P<0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test.
| Substance | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|
| 2-Chlorobenzoic acid | 2.05 | 10 | -0.007 ± 0.019 | |
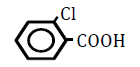 |
25 | -0.026 ± 0.021 | ||
| 50 | -0.047 ± 0.011 | |||
| 100 | -0.094 ± 0.018 | ** | ||
| 3-Chlorobenzoic acid | 2.65 | 10 | -0.020 ± 0.011 | |
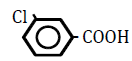 |
25 | -0.051 ± 0.027 | * | |
| 50 | -0.093 ± 0.019 | ** | ||
| 100 | -0.155 ± 0.051 | ** | ||
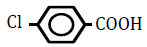
| 4-Chlorobenzoic acid | 2.65 | 10 | -0.026 ± 0.027 | |
 |
25 | -0.061 ± 0.027 | * | |
| 50 | -0.104 ± 0.026 | ** | ||
| 100 | -0.116 ± 0.021 | ** | ||
| 2-Bromobenzoic acid | 2.2 | 10 | -0.012 ± 0.018 | |
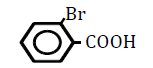 |
25 | -0.027 ± 0.021 | ||
| 50 | -0.036 ± 0.029 | |||
| 100 | -0.081 ± 0.031 | * | ||
| 3-Bromobenzoic acid | 2.87 | 10 | -0.031 ± 0.018 | |
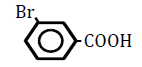 |
25 | -0.058 ± 0.023 | ||
| 50 | -0.091 ± 0.028 | ** | ||
| 100 | -0.116 ± 0.024 | ** | ||
| 4-Bromobenzoic acid | 2.86 | 10 | -0.025 ± 0.017 | |
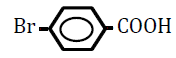 |
25 | -0.064 ± 0.033 | ** | |
| 50 | -0.101 ± 0.012 | ** | ||
| 100 | -0.121 ± 0.018 | ** | ||
Table 3: Effects of benzoic acid derivatives in which hydrogen was replaced with a chlorine atom or a bromine atom on the benzene ring on OF in sheep RBCs. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [21] website. The ΔEC50 values (amount of change in NaCl %) at 10, 25, 50 and 100 mM are presented. Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test.
| Substance | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|
| 2,3-Dihydroxy benzoic acid | 1.2 | 10 | -0.017 ± 0.022 | |
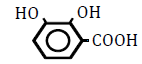 |
25 | -0.033 ± 0.014 | ||
| 50 | -0.063 ± 0.015 | * | ||
| 100 | -0.117 ± 0.023 | ** | ||
| 2,4-Dihydroxoy benzoic acid | 1.63 | 10 | -0.013 ± 0.011 | |
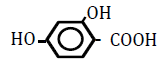 |
25 | -0.027 ± 0.007 | ||
| 50 | -0.054 ± 0.018 | |||
| 100 | -0.074 ± 0.029 | * | ||
| 2,5-Dihydroxoy benzoic acid | 1.74 | 10 | -0.017 ± 0.013 | |
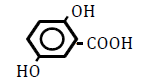 |
25 | -0.033 ± 0.023 | ||
| 50 | -0.048 ± 0.021 | |||
| 100 | -0.087 ± 0.023 | ** | ||
| 2,6-Dihydroxoy benzoic acid | 2.20 | 10 | -0.021 ± 0.018 | |
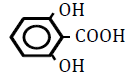 |
25 | -0.061 ± 0.040 | ||
| 50 | -0.078 ± 0.009 | * | ||
| 100 | -0.115 ± 0.017 | ** | ||
| 3,4-Dihydroxoy benzoic acid | 0.86 | 10 | 0.013 ± 0.021 | |
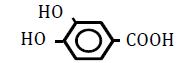 |
25 | 0.018 ± 0.015 | ||
| 50 | -0.003 ± 0.023 | |||
| 100 | -0.028 ± 0.025 | |||
| 3,5-Dihydroxoy benzoic acid | 0.86 | 10 | 0.002 ± 0.016 | |
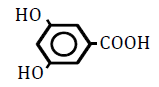 |
25 | -0.006 ± 0.019 | ||
| 50 | -0.027 ± 0.029 | |||
| 100 | -0.060 ± 0.034 | ** | ||
Table 4: Effects of benzoic acid derivatives in which hydrogen was replaced with hydroxy groups at two positions on the benzene ring on OF in sheep RBCs. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [21] website. The ΔEC50 values (amount of change in NaCl %) at 10, 25, 50 and 100 mM are presented. Asterisks (* and **) indicate that there was a significant difference (P<0.05 and P<0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test.
| Substance | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|
| 2,3-Dichlorobenzoic acid | 2.92 | 10 | -0.013 ± 0.015 | |
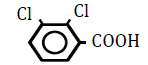 |
25 | -0.022 ± 0.011 | ||
| 50 | -0.035 ± 0.016 | |||
| 100 | -0.041 ± 0.020 | |||
| 2,4-Dichlorobenzoic acid | 2.81 | 10 | -0.014 ± 0.014 | |
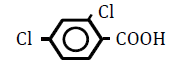 |
25 | -0.045 ± 0.023 | * | |
| 50 | -0.062 ± 0.031 | ** | ||
| 100 | -0.071 ± 0.018 | ** | ||
| 2,5-Dichlorbenzoic acid | 2.97 | 10 | -0.005 ± 0.011 | |
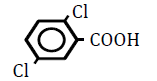 |
25 | -0.007 ± 0.015 | ||
| 50 | -0.024 ± 0.014 | |||
| 100 | -0.039 ± 0.016 | |||
| 2,6-Dichlorobenzoic acid | 2.20 | 10 | -0.002 ± 0.015 | |
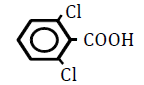 |
25 | -0.012 ± 0.018 | ||
| 50 | -0.017 ± 0.023 | |||
| 100 | -0.033 ± 0.025 | |||
| 3,4-Dichlorobenzoic acid | 3.53 | 10 | -0.011 ± 0.009 | |
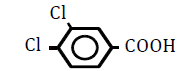 |
25 | -0.051 ± 0.019 | ||
| 50 | -0.086 ± 0.032 | ** | ||
| 100 | No data (burst) | |||
| 3,5-Dichlorobenzoic acid | 3.92 | 10 | -0.038 ± 0.015 | |
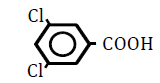 |
25 | -0.084 ± 0.022 | ** | |
| 50 | -0.097 ± 0.029 | ** | ||
| 100 | No data (burst) | |||
Table 5: Effects of benzoic acid derivatives in which hydrogen was replaced with chlorine atoms at two positons on the benzene ring on OF in sheep RBCs. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [32] or ChemSpider [21] website. The ΔEC50 values (amount of change in NaCl %) at 10, 25, 50 and 100 mM are presented. Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test.
Replacement of the benzene ring or carboxylic group by other elements
Figure 1 shows changes in OF in sheep RBCs by the application of benzoic acid (C6H5-COOH) and its related substances, in which the benzene ring (C6H5) or a carboxylic group (COOH) was replaced by other elements. Benzoic acid decreased OF in a dose-dependent manner (P<0.05), whereas heptanoic acid (C6H13-COOH), which has 6 carbons in its straight hydrocarbon chain, did not affect OF in sheep RBCs (Figure 1A). The effects of the replacement of the carboxylic group (COOH) by another group, (PO(OH)2 ) or (CONH2), are shown in Figure 1B. Benzenephosphonic acid (C6H5-PO(OH)2) tended to increase OF at 10 and 25 mM and decrease OF at 100 mM, but these changes are not statistically significant (N.S). Benzamide (C6H5-CONH2) decreased OF in a dose-dependent manner from 2.5 to 100 mM. The ΔEC50 values of all tested substances, including cyclohexanecarboxylic acid (C6H11-COOH), hydroxybenzene (C6H5- OH) and benzenesulfonic acid (C6H5-SO2OH), are summarized in Table 1. Cyclohexanecarboxylic acid and benzenesulfonic acid did not affect OF in sheep RBCs at 10 to 100 mM. Hydroxybenzene did not affect OF below 50 mM, but induced abrupt hemolysis at 50 and 100 mM.
Replacement of a hydrogen on the benzene ring by other groups
Replacement of a hydrogen (H) by a hydroxy (OH) or methyl (CH3) group at position 2 or 4 on the benzene ring decreased OF in sheep RBCs (Figure 2A and B). Both 2- and 4-hydroxybenzoic acid significantly decreased OF in a dose-dependent manner (P<0.05). Although 2-methylbenzoic acid did not affect OF, 4-methylbenzoic acids significantly decreased OF in a dose-dependent manner (P<0.05). The ΔEC50 values of all tested substances, including 3-hydroxybenzoic acid, 3-metylbenzoic acid and 3 isomers of aminobenzoic acid (C6H5- NH2), are summarized in Table 2. Statistically significant decreases in OF were obtained by 3-hydroxybenzoic acid at 100 mM and 3-metylbenzoic acid at 25, 50 and 100 mM (P<0.05). Among the 3 isomers of aminobenzoic acid, statistically significant decreases in OF (P<0.05) were observed for 2-aminobenzoic acid at 50 and 100 mM, and 4-aminobenzoic acid at 100 mM, but not for 3-aminobenzoic acid at 10 to 100 mM.
Replacement of a hydrogen on the benzene ring by a halogen
Replacement of a hydrogen (H) on the benzene ring by a chlorine (Cl) or bromine (Br) atom at position 2 or 4 led to dose-dependent decreases in OF in sheep RBCs (P<0.05) (Figure 3A and B). The degree of the decrease in OF was dependent on the position of the substitution for both chlorobenzoic acids and bromobezoic acids. The ΔEC50 values of all tested substances, including 3-chlorobenoic acid and 3-bromobenzoic acid, are summarized in Table 3. Statistically significant decreases in OF (P<0.05) were observed for the application of 3-chlorobenoic acid at 10, 25 and 50 mM, and 3-bromobenzoic acid at 50 and 100 mM.
Replacement of hydrogens at two positions on the benzene ring by a hydroxyl group
The four isomers of dihydroxybenzoic acid, which have 2 hydroxy groups (OH) at different positions on the benzene ring, were examined (Figure 4A and B). 2,3-, 2,4- and 2,6-Hydroxybenzoic acid decreased OF (P<0.05), but 3,4-hydroxybenzoic acid only tended to decrease OF (N.S). The degree of the decreases in OF depended on the position of the substitution on the benzene ring. The ΔEC50 values of all tested substances, including 2,5-hydroxybenoic acid and 3,5-hydroxybenoic acid, are summarized in Table 4, Both 2,5- and 3,5-hydroxybenoic acids significantly decreased OF dose-dependently at 100 mM application (P<0.05). Again, the degree of the decrease in OF was dependent on the positions of hydroxy groups on the benzene ring.
Replacement of hydrogens at two positions on the benzene ring by a chlorine
The four isomers of dichlorobenzoic acid, which have 2 chlorine atoms (Cl) at different positions on the benzene ring, were examined (Figure 5A and B). Although 2,3- and 2,6-dichlorobenzoic acids tended to decrease OF, these changes were N.S. 2,4-Dichlorobenzoic acid decreased OF at concentration from 25 to 100 mM (P<0.05), whereas 3,4-dichlorobenzoic acid decreased OF at 25 and 50 mM (P<0.05) and then induced hemolysis at 100 mM. The ΔEC50 values of all tested substances, including 2,5-dichlorobenoic acid and 3,5-dichlorobenoic acid, are summarized in Table 5. Although 2,5-dichlorobenoic acid tended to decrease OF at 50 and 100 mM, these changes were N.S. 3,5-Dichlorobenoic acid decreased OF at 25 and 50 mM (P<0.05) and then induced hemolysis at 100 mM. The degree of the decrease in OF was dependent on the positions of the chlorine atoms on the benzene ring.
Relationship between partition coefficient and change in OF
Table 6 shows the results of the regression analysis between the partition coefficients of the substances and their effects on OF. Analyses were performed for each group of substances, all tested substances and benzoic acid plus substances in this experiment. There is no obvious correlation between their partition coefficients and changes in OF for benzoic acid and the group of derivatives in which the benzene ring (C6H5) or carboxylic base (COOH) was substituted with another structure or in which OH, CH3 or NH2 was introduced on the benzene ring. For the derivatives with a halogen substitution, negative high correlations were observed between the partition coefficients and decreases in OF at concentration of 10, 25 and 50 mM (P<0.01). A negative correlation (P<0.05) was also observed for the derivatives in which H was substituted with OH or Cl at two positions on the benzene ring at concentration of 25 and 50 mM. The analysis of 28 substances including benzoic acid in Table 1 and other derivatives in Tables 2-5 revealed a negative correlation between the partition coefficients and decreases in OF at concentrations of 10, 25 and 50 mM (P<0.01), but not 100 mM. The analysis of all substances (n=34) used in this study showed a statistically significant correlation between the partition coefficients and the decreases in OF at 50 mM, but not at any other concentration.
| Analysis | n | mM | Intercept | Slope | r | P | |
|---|---|---|---|---|---|---|---|
| Table 1 | 7 | 10 | -0.0091 | 0.0053 | 0.2538 | 0.5829 | |
| 7 | 25 | -0.0117 | 0.0058 | 0.2424 | 0.6005 | ||
| 6 | 50 | -0.0226 | 0.0010 | 0.0541 | 0.9190 | ||
| 6 | 100 | -0.0329 | -0.0050 | 0.1957 | 0.7102 | ||
| Table 2 | 9 | 10 | 0.0000 | -0.0064 | 0.5271 | 0.1448 | |
| 9 | 25 | -0.0031 | -0.0152 | 0.5155 | 0.1555 | ||
| 9 | 50 | -0.0077 | -0.0293 | 0.4887 | 0.1819 | ||
| 8 | 100 | -0.0532 | -0.0056 | 0.1731 | 0.6819 | ||
| Table 3 | 6 | 10 | 0.0444 | -0.0253 | 0.9563 | 0.0028 | ** |
| 6 | 25 | 0.0733 | -0.0476 | 0.9573 | 0.0027 | ** | |
| 6 | 50 | 0.1179 | -0.0772 | 0.9024 | 0.0138 | * | |
| 6 | 100 | 0.0097 | -0.0485 | 0.6562 | 0.1569 | ||
| Table 4 | 6 | 10 | 0.0203 | -0.0206 | 0.8248 | 0.0434 | |
| 6 | 25 | 0.0396 | -0.0447 | 0.8882 | 0.0180 | * | |
| 6 | 50 | 0.0121 | -0.0407 | 0.8129 | 0.0493 | * | |
| 6 | 100 | -0.0204 | -0.0423 | 0.6648 | 0.1497 | ||
| Table 5 | 6 | 10 | 0.0383 | -0.0170 | 0.8005 | 0.0557 | |
| 6 | 25 | 0.0850 | -0.0398 | 0.8204 | 0.0455 | * | |
| 6 | 50 | 0.0955 | -0.0487 | 0.8735 | 0.0230 | * | |
| 6 | 100 | -0.0012 | -0.0164 | 0.3443 | 0.6557 | ||
| Table 1-5 | 34 | 10 | -0.0022 | -0.0046 | 0.3075 | 0.0768 | |
| 34 | 25 | -0.0025 | -0.0132 | 0.4923 | 0.0031 | ** | |
| 34 | 50 | -0.0188 | -0.0171 | 0.4940 | 0.0035 | ** | |
| 34 | 100 | -0.0427 | -0.0145 | 0.3585 | 0.0517 | ||
| Benzoic acid + Table 2-5 | 28 | 10 | 0.0045 | -0.0081 | 0.6106 | 0.0006 | ** |
| 28 | 25 | 0.0054 | -0.0182 | 0.6460 | 0.0002 | ** | |
| 28 | 50 | -0.0140 | -0.0207 | 0.5160 | 0.0049 | ** | |
| 25 | 100 | -0.0487 | -0.0143 | 0.3121 | 0.1287 |
Table 6: Correlation between the partition coefficients of carboxylic acids and changes in EC50 during hemolysis in sheep RBCs. Values were calculated by regression analysis (mean value of each substance) between the partition coefficients and ΔEC50 values during hemolysis induced by each dose of benzoic acid and its derivatives. The analysis was done on the group of substances in each of Table 1-5, all substances (Table 1-5), and benzoic acid plus substances in Table 2-5. Correlation efficient “r” and significance “P”are shown. P < 0.05 or P<0.01 is defined as statistically significant in the present study.
In this series of experiments, we found that benzoic acid and most of its derivatives decreased OF in isolated sheep RBCs. The significant decreases in OF occurred in a dose-dependent manner and were dependent on the chemical structure of the derivatives.
Replacement of the benzene ring (C6H5) with a straight hydrocarbon chain (C6H13) or cyclohexane ring (C6H11), both of which possess 6 carbons in their structure, abolished the benzoic acid-induced decrease in OF. The replacement of the carboxylic group (COOH) with other groups induced various degree of change in OF. Although hydroxybenzene (OH) did not change OF at concentrations from 0.1 to 25 mM, it induced hemolysis at 50 and 100 mM. Benzenephosphonic acid (PO(OH)2) and benzenesulfonic acid (SO2OH) abolished the benzoic acid-induced decrease in OF. Benzamide (CONH2) markedly lowered OF and the degree of the decrease in OF induced by benzamide was much larger than that induced by benzoic acid.
Replacement of a hydrogen (H) atom on the benzene ring with another groups (OH, CH3 or NH3) or a halogen (Cl or Br) also decreased or tended to decrease OF. The degree of the decrease in OF depended on the type of element introduced position on the benzene ring at which the substitution occurred. 4-Metyhlbenzoic acid decreased OF at 25 and 50 mM and induced hemolysis at 100 mM. The derivatives in which OH groups were introduced at two positions on the benzene ring induced decreases in OF. Most derivatives in which two Cl atoms were introduced on the benzene ring decreased or tended to decrease OF dose-dependently. 3.4- and 3.5-Dichlorobenzoic acids, however, decreased OF at concentrations up to 50 mM and then both derivatives induced hemolysis at the highest concentration of 100 mM.
The results of this study also confirmed the relationship between the partition coefficients of benzoic acid and its derivatives, and their effects on OF. Partition coefficients have been widely used as indicators for the delivery of various chemicals into cells, tissues and the body [17- 20]. The octanol/water partition coefficients are commonly employed for evaluating the permeation of substances into the membrane, as the physicochemical characteristics of octanol are closer in nature to those of phospholipids [27]. For the regression analysis of the effect of benzoic acid and its derivatives on OF in sheep RBCs, we used the octanol/water partition coefficients quoted from the three websites mentioned above [24-26].
For the derivatives in which the basic structural elements such as the benzene ring or carboxylic group were replaced with other structures or those in which H atoms were replaced with OH, NH3 or NH2 on the benzene ring, no correlations were observed between their partition coefficients and changes in OF. On the other hand, in most derivatives in which a halogen atom (Cl or Br) was introduced on the benzene ring or two OH or Cl substitution were made on the benzene ring, significantly negative correlations were observed between their partition coefficients and the induced decreases in OF at concentrations of 10-50 mM or 25 and 50 mM, respectively.
As some derivatives also demonstrated a hemolytic effect at a concentration of 100 mM in sheep RBCs. we considered that the OF response becomes unstable in the presence of certain substances at a concentration approaching 100 mM and the fluctuation range of the OF value also increases. This is because no significant correlation between the partition coefficients and changes in OF could be obtained at the highest concentration of substances. It is difficult to comprehensively evaluate the effect of all benzoic acid-related substances with a broad ranges of structure on OF in sheep RBCs by the partition coefficients of these substances. However, for substances sharing very close chemical structures, such as derivatives with a Cl atom introduced at position 2, 3 or 4 on the benzene ring, the partition coefficients can potentially be used as an indicator of their effect on OF within an appropriate range of concentrations.
Among the tested benzoic acid derivatives, 4-methylbenzoic acid, and 3,4- and 4,5-dichlorobenzoic acids demonstrated a biphasic or rebound effect on OF in sheep RBCs; decreasing OF (or protecting hypotonic hemolysis) at lower concentrations up to 50 mM and inducing hemolysis at the highest concentration of 100 mM. Although, in our previous experiments, the same biphasic changes in OF in response to benzoic acid and its derivatives were demonstrated in guinea pig RBCs [15], these changes were not observed in rat RBCs [14]. This protective and lytic behavior on the RBC membrane has been reported in various surfactants [28-31].
We have proposed that this stabilizing effect on the RBC membrane against osmotic lysis by dicarboxylic acids as a “wedge-like effect” [16,32]. The concept of the wedge-like effect on the membrane has already proposed in amphipathic alpha helixes in apolipoprotein, having a large three-dimensional structure [33]. Different to that concept, in our proposed concept, much smaller substances such as dicarboxylic acids develop a stabilizing effect on the cell membrane [16,32]. Dicarboxylic acids have two hydrophilic carboxylic groups situated at either end of their hydrophobic hydrocarbon chain. As the two hydrophilic carboxylic groups cannot enter deeply into hydrophobic acyl-chain layer, these elements are positioned at lipid-water interface of the phospholipid layer directed toward outer water area. The hydrophobic hydrocarbon chain is thought to enter the region in which the roots of the acyl-chains are combined the phospholipid heads. Under these conditions, the hydrophilic hydrocarbons of the dicarboxylic acids cannot penetrate far into the hydrophobic acyl-chain region in the phospholipid layer. This wedge-like effect of dicarboxylic acids was demonstrated in rat [16,32], guinea pig [16] and sheep RBCs [34].
Benzoic acid and its related substances ware also speculated to permeate the RBC membrane, interact with phospholipids and increase resistance to low osmotic pressure in the outer solution. Two possibilities are currently proposed to explain the detailed mechanism underlying protective effect of amphiphilic substances against hypotonic hemolysis after the permeation of those substances [35]. Various amphiphilic substances including surfactants, anesthetics, and so on have been reported to increase cell volume or membrane area in RBCs [36-38]. It has been speculated that the swelling of RBCs is the reason for protecting effect against hemolysis. On the other hand, it has also been reported that the protective effect of amphiphilic substances against hemolysis occurs without swelling of the RBCs [39,40]. The permeation of substances into the RBC membrane leads to changes in the ion balance between inside and outside of the RBC membrane, thereby protecting against hypotonic hemolysis [41,42]. It was reported that cell lysis through swelling or changes in the ion balance is dependent on the type of substances involved [43].
Although OF was decreased by most dicarboxylic acids [14-16,32,34] and some monocarboxylic acids [9,14,15,47], the mechanism underlying this effect may differ between two types of carboxylic acids. Monocarboxylic acids are considered to be surfactants as they show amphiphilic characteristics. Some surfactants are known to affect the cell membrane in a biphasic manner; showing a stabilizing effect at low and a lytic effect at high concentrations [28-31]. Dicarboxylic acids have lower partition coefficients than the corresponding monocarboxylic acids, and the site of action of the dicarboxylic acids is thought to be shallow area of the RBC membrane or water-lipid interface and that of the monocarboxylic acids is considered to be the deeper area of the RBC membrane [44,45]. Thus, the decrease in OF induced by some benzoic acid derivatives may represent the surfactant stabilizing effect when applied at low concentrations as part of the biphasic effect observed for amphiphilic agents. The mechanism underlying the reduction in OF by monocarboxylic acids and dicarboxylic acids, including the site of action in the RBC membrane, needs to be clarified in further experiment.
RBCs were used as a prototypical cellular model with which to examine chemical-mediated effects on the plasma membrane. However, our series of experiments demonstrated that benzoic acid and its derivatives increased OF in rat RBCs [14] decreased OF in guinea pig RBCs [15] and also decreased OF in sheep RBCs, as demonstrated in this series of experiments. These inter-species differences have been observed in the OF response to monocarboxylic acid having straight hydrocarbon chains in rat [13,16], guinea pig [16] and sheep RBCs [34]. These differences in OF response in RBCs among species are speculated to be based on differences in the phospholipid composition of the RBC membrane among species [16], as there have been many reports that the phospholipid composition of erythrocytes appear to differ among species [46-50].
In our series of experiments mentioned above, we focused on the different proportions of arachidonic acid in the phospholipid composition of rat, guinea pig and sheep RBCs [49,50]. The reason is that the proportion of arachidonic acid in the rat RBC membrane is about 30% or more [50,51], which is much larger than that in the guinea pig [49] and sheep RBC membrane [50]. Arachidonic acid is a polyunsaturated fatty acid with a crooked hydrocarbon chain due to four unsaturated carbon bonds in its moiety. This molecular structure is speculated to disturb the rigid binding of acyl-chains aligned linearly in the phospholipid layer of the cell membrane.
We have recently proposed a model of the action induced by carboxylic acid-related substances, including benzoic acid, which is based on the differences in the fatty acid composition of the RBC membrane among rats, guinea pigs and sheep [34]. In cell membranes with a higher proportion of arachidonic acid, such as in rat RBCs, benzoic acid and its derivatives are speculated to invade the acyl-chain matrix, which has loose bonds and a certain amount of free space, in the phospholipid layer [34]. Those substances enter deeply into the RBC membrane, inducing partial lysis of the RBC membrane and thereby increasing OF in rat RBCs. On the other hand, benzoic acid and its derivatives cannot enter deeply into the membrane and stay in shallower region of the water-lipid interface, making stable connections between the phospholipids and increasing osmotic resistance in the guinea pig and sheep RBCs.
The protection against and reduction in membrane resistance to osmotic pressure induced by carboxylic acids, including benzoic acid and its derivatives, are interesting issues with the relationship between the phospholipid composition and OF response in the RBC membrane, in particular, requiring more detailed clarification. As amphiphilic substances permeating into the cell membrane affect various physiological cell behaviors such as endocytosis [51,52], membrane protein movement [53,54], and cell aggregation [55,56], benzoic acid and its derivatives are also expected to have the potential to affect functions in various cells. Further experiments using RBCs in various animals other than rats, guinea pigs and sheep are needed to clarify the discrepant effect of carboxylic acids, including benzoic acid and its derivatives, on osmotic resistance in RBCs.
We would like to thank the technical staff at the Ecorin Village Farm (Eniwa, Hokkaido) for sampling blood from sheep and providing these samples to our laboratory.