Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Research Article - (2023)Volume 11, Issue 7
Aim: The aim of this study is to investigate the impact of beta thalassemia on lipid profiles, nitric oxide levels, and cardiovascular health. Through comprehensive analysis, the study seeks to uncover the intricate relationships between these factors, contributing to a better understanding of the cardiovascular complications associated with beta thalassemia and providing insights for potential therapeutic strategies.
Objective: This study aims to explore the connection between beta thalassemia, lipid profiles, nitric oxide levels, and cardiovascular risk factors. The goal is to enhance understanding of the multifaceted mechanisms underlying cardiovascular complications in beta thalassemia patients undergoing chelation therapy.
Method: A cohort of thalassemia major patients (ages 1.5 to 30) undergoing chelation therapy was selected. Blood samples were collected, and serum was analyzed for lipid profiles, calcium, phosphorus, and NO levels. Detailed patient histories and treatment details were recorded. Commercial analytical kits were used for analyses, and statistical tests were performed to compare results.
Results and discussion: In thalassemia major patients, altered lipid profiles were observed, characterized by decreased Total Cholesterol (TC), High-Density Lipoprotein Cholesterol (HDL-C), Low-Density Lipoprotein Cholesterol (LDLC), and Very-Low-Density Lipoprotein Cholesterol (VLDL-C) levels. However, Triglyceride (TG) levels showed discrepancies. Iron overload, ineffective erythropoiesis, accelerated erythropoiesis, liver damage, reduced enzyme activity, and hormonal imbalances contributed to these lipid abnormalities.
Conclusion: Beta thalassemia patients face altered lipid profiles and reduced NO levels, elevating their risk of coronary complications. Iron overload-induced oxidative stress is a key contributor. Early initiation of iron chelation and antioxidants is crucial. Comprehensive cardiovascular risk assessment in these patients is essential to mitigate potential coronary issues, improving their overall well-being and quality of life. This review sheds light on the intricate links among beta thalassemia, lipid profiles, NO levels, and cardiovascular health, emphasizing the need for multifaceted interventions.
Beta thalassemia; Chelation therapy; Lipid abnormalities; Cardiovascular health
In the realm of inherited blood disorders, Beta Thalassemia stands as a significant clinical challenge with multifaceted implications. This hereditary condition, characterized by reduced or absent synthesis of beta-globin chains of hemoglobin, disrupts the delicate equilibrium within the bloodstream and poses a spectrum of health concerns. Among the manifold consequences of Beta Thalassemia, its influence on lipid profile, nitric oxide metabolism, and cardiovascular health has garnered substantial attention in recent medical research [1]. These interrelated aspects intricately contribute to the overall clinical picture of Beta Thalassemia, underscoring the necessity for comprehensive understanding and effective management.
Beta Thalassemia, a genetic disorder stemming from mutations in the HBB gene, disrupts the production of beta-globin chains and subsequently affects the synthesis of hemoglobin, the vital oxygen-carrying molecule in red blood cells. The resultant imbalanced globin chain production engenders anemia, a cardinal feature of this disorder, necessitating regular blood transfusions to ameliorate oxygen transport [2]. However, the intricate interplay between Beta Thalassemia and other physiological processes extends beyond erythropoiesis, unveiling an intricate web of connections that reach into lipid metabolism and cardiovascular well-being.
Alterations in lipid profile, including variations in cholesterol and triglyceride levels, have been identified in individuals with Beta Thalassemia. While the exact mechanisms driving these changes are still being deciphered, chronic anemia and iron overload resulting from frequent blood transfusions likely play pivotal roles. Elevated levels of Low-Density Lipoprotein Cholesterol (LDL-C) and triglycerides, coupled with decreased levels of High-Density Lipoprotein Cholesterol (HDL-C), have been observed in certain Beta Thalassemia patients [3-5]. These lipid abnormalities not only contribute to the heightened risk of atherosclerosis but also shed light on the intricate interplay between iron metabolism, erythropoiesis, and lipid homeostasis.
A particularly intriguing facet of Beta Thalassemia's impact on cardiovascular health lies in its influence on Nitric Oxide (NO) metabolism. Nitric oxide, a potent vasodilator and signaling molecule, exerts crucial functions in maintaining vascular tone, regulating blood pressure, and inhibiting platelet aggregation. Emerging evidence suggests that Beta Thalassemia patients may experience reduced bioavailability of nitric oxide due to factors such as oxidative stress, endothelial dysfunction, and inflammation. This impairment not only contributes to endothelial dysfunction and vascular complications but also implicates nitric oxide in the intricate nexus between anemia, iron overload, and cardiovascular health in Beta Thalassemia.
The convergence of these factors underscores the comprehensive nature of the challenges posed by Beta Thalassemia. The intricate relationships between altered lipid profiles, compromised nitric oxide metabolism, and cardiovascular health not only shed light on the mechanisms underlying the increased cardiovascular risk in this population but also present potential avenues for therapeutic interventions. Strategies aimed at mitigating iron overload, modulating lipid metabolism, and enhancing nitric oxide bioavailability hold promise for ameliorating the cardiovascular burden faced by Beta Thalassemia patients [6].
The impact of Beta Thalassemia on lipid profile, nitric oxide metabolism, and cardiovascular health transcends the traditional boundaries of hematology and branches into broader domains of cardiovascular medicine. The intricate interconnections between altered lipid profiles, nitric oxide dysfunction, anemia, and iron overload create a complex clinical landscape that demands holistic understanding and innovative therapeutic approaches. As ongoing research delves deeper into the underlying mechanisms, novel strategies may emerge, offering hope for improving the quality of life and overall health outcomes for individuals living with Beta Thalassemia.
The study focused on patients with thalassemia who were undergoing regular chelation therapy. The cohort consisted of females and males, all of whom were free from HBV, HCV and HIV. Informed consent was obtained, and blood samples were collected after fasting in red vacutainers. For minors, consent was obtained from parents [7].
The blood samples were allowed to clot and serum was separated through centrifugation. The samples were analyzed on the same day for various parameters in lipid profile like cholesterol, triglycerides, HDL, LDL and calcium, phosphorus, and nitric oxide levels (Figure 1). Detailed patient histories were recorded, encompassing aspects such as thalassemia history, initiation of blood transfusions, frequency of transfusions per month, and chelation therapy details.
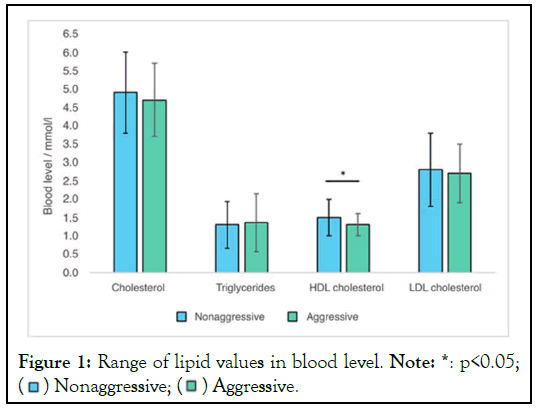
Figure 1: Range of lipid values in blood level. Note: *: p<0.05; 
Commercial analytical kits from Randox were employed to evaluate calcium, phosphorus, Triglycerides (TG), Total Cholesterol (TC), High-Density Lipoprotein Cholesterol (HDLC), Low-Density Lipoprotein Cholesterol (LDL-C) and Very- Low-Density Lipoprotein Cholesterol (VLDLC). The Nitric Oxide (NO) levels, measured as Nitrite Plus Nitrate (NO(x)) concentration, were determined using the Griess reagent method.
The statistical analysis included calculating average values in Table 1. The comparison between Thalassemia patients with controlled group by performed t-tests, with a significance level of *p<0.05 considered as significant and p<0.001 considered as statistically highly significant. This study aimed to shed light on the relationship between thalassemia, lipid profiles, calcium, phosphorus, and nitric oxide levels, contributing to a better understanding of cardiovascular risk factors in thalassemia major patients undergoing chelation therapy.
| Variables | Thalassemia patients | Control group | p-value |
|---|---|---|---|
| calcium | 106.38-1288.88 | 271.8-1187 | p<0.001 |
| phosphorus | 33.2-9800 | 108-1510.3 | p<0.001 |
| triglycerides | 3.88-8.33 mmol/L | 7.44(4.1) mmol/L | p<0.01 |
| total cholesterol | 25-55.5 mmol/L | 26.6(6.8) mmol/L | P<0.0001 |
| HDL-C | ≥ 1.94 mmol/L | 1.0 (0.7) mmol/L | p>0.05 |
| LDL-C | ≤ 8.33 mmol/L | 2.19(1.7) mmol/L | p>0.05 |
| NO | 34.845 ± 6.249 | 48.613 ± 17.359 | p<0.001 |
| NO(x) | 42.548 ± 7.379 | 85.975 ± 35.286 | p<0.001 |
Table 1: Statistical analysis between thalassemia patients and control group.
Beta thalassaemia major is a prevalent genetic disorder among tribal populations in India. A study was conducted to investigate the lipid profile and Nitric Oxide (NO) levels in beta thalassemia patients compared to matched healthy controls. The research revealed lower levels of TC, HDL-C, LDL-C and VLDL-C in thalassemia patients [1,8]. These findings were consistent with previous studies by various authors. However, TG levels were higher in the cases, showing discrepancies among different studies.
The altered lipid profile in thalassemia patients can be attributed to multiple factors. Iron overload resulting from transfusions and chelation therapy damages organs, particularly the liver, heart, and endocrine glands, due to iron-induced free radical damage. Ineffective erythropoiesis and accelerated erythropoiesis in beta thalassemia contribute to lipid abnormalities. Liver damage and reduced enzyme activity further affect lipoprotein metabolism. Hormonal imbalances also play a role.
The decrease in HDL-C levels in thalassemia patients increases the risk of Myocardial Ischemia (MI), emphasizing the need for cardiovascular risk assessment. The TC/HDL-C ratio is suggested as a more informative marker than absolute values [9]. The oxidative stress resulting from chronic hemolysis in hemoglobinopathies leads to endothelial dysfunction and reduction of NO [10]. NO's interaction with Reactive Oxygen Species (ROS) further exacerbates oxidative damage and promotes vasoconstriction and platelet aggregation, posing a risk for MI. The uncoupling of Endothelial Nitric Oxide Synthase (eNOS) under increased oxidative stress contributes to decrease NO production.
In the intricate tapestry of Beta Thalassemia, the interplay between altered lipid profiles and diminished nitric oxide levels serves as a clarion call to recognize the heightened risk of cardiovascular complications among afflicted individuals. This risk is grounded in the disease's nexus with oxidative stress, a consequence primarily of iron overload. The surplus iron sets the stage for lipid peroxidation, casting a shadow over cardiovascular health. Timely intervention through iron chelation therapies and antioxidants becomes paramount, offering a glimmer of hope in preventing the cascade of events that could lead to coronary complications. Furthermore, an astute assessment of cardiovascular risk factors must be an integral facet of managing beta thalassemia patients, imparting the knowledge needed to navigate this complex terrain and ultimately attenuate the potential for severe coronary issues. The confluence of altered lipids, dwindling nitric oxide, oxidative stress, and iron overload, we unlock a pathway towards enhanced patient care. By arming ourselves with knowledge, embracing a holistic perspective, and fostering collaboration between medical experts, we can reshape the trajectory of beta thalassemia management.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Uddin M, Khaleque A (2023) Beta Thalassemia: Impact on Lipid Profile, Nitric Oxide, and Cardiovascular Health. J Hematol Thrombo Dis. 11:557.
Received: 03-Jul-2023, Manuscript No. JHTD-23-26147; Editor assigned: 05-Jul-2023, Pre QC No. JHTD-23-26147 (PQ); Reviewed: 19-Jul-2023, QC No. JHTD-23-26147; Revised: 26-Jul-2023, Manuscript No. JHTD-23-26147 (R); Published: 02-Aug-2023 , DOI: 10.35248/2329-8790.23.11.557
Copyright: © 2023 Uddin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.