Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research - (2019)Volume 9, Issue 4
The non-alcoholic steatohepatitis (NASH) increases the cardiovascular risk regardless of risk factors in metabolic syndrome. The stroke-prone (SP) spontaneously hypertensive rats (SHRSP5/Dmcr), fed a high-fat and -cholesterol (HFC) diet and administered Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) presented NASH and cardiovascular disease. However, the intermediary factors between NASH and cardiovascular risk are still unknown. We investigated whether bile acids (BAs) play an intermediary role between NASH and cardiovascular disease in SHRSP5/Dmcr rats. SHRSP5/Dmcr rats divided into 2 groups were fed an SP (non-NASH group, n=7) or HFC diet (NASH group, n=10) for 8 weeks. L-NAME was administered intraperitoneally in the final 2 weeks. In the NASH group, total BA levels were increased in both serum and liver; they aggravated the endothelial dysfunction in aorta, which is the first step to atherosclerosis, and activated the gene expression levels of cell adhesion molecules (vascular cell adhesion molecule-1, intercellular adhesion molecule-1) in coronary artery. In particular, the nuclear factor-kappa B (NFkB) was increased in the NASH group in the coronary endothelial cells activated by high BA levels. We demonstrated that liver BAs aggravated steatosis and fibrosis and serum BA accelerated arteriosclerosis and ischemic heart disease via the classical pathway of NFkB in the endothelial cells. BA metabolism disorder may be an intermediary factor between NASH and cardiovascular risk in SHRSP5/Dmcr rats.
Bile acids; Non-alcoholic steatohepatitis; SHRSP5/Dmcr; Atherosclerosis
Non-alcoholic fatty liver disease (NAFLD) is strongly related to obesity, diabetes and other lifestyle-related diseases, and is a common cause of chronic liver disease with increasing worldwide prevalence. NAFLD consists of a broad spectrum of diseases, ranging from simple steatosis (Non-alcoholic fatty liver: NAFL) without hepatocellular injury to non-alcoholic steatohepatitis (NASH) marked by inflammation and hepatocyte injury with or without fibrosis [1]. Recently, NAFLD/NASH patients exhibit a high risk for cardiovascular disease, independent of the broad spectrum of risk factors in the metabolic syndrome [2,3].
Retrospective and prospective studies indicated evidence of strong relationships between NASH and subclinical manifestations of atherosclerosis, as increased intima-media thickness, endothelial dysfunction, arterial stiffness, impaired left ventricular (LV) function, and coronary calcification [4]. However, the intermediary factors between NASH and cardiovascular risk, with exceptions of insulin resistance and oxidative stress, are still unknown because suitable animal models have not been established yet.
In previous reports, we demonstrated that stroke-prone (SP) spontaneously hypertensive rats (SHRSP5/Dmcr) fed a high-fat and -cholesterol (HFC) diet presented NASH pathology and showed cardiac or vascular dysfunction, such as cardiac fibrosis, endothelial dysfunction, and left ventricle (LV) diastolic dysfunction [5]. Furthermore, the SHRSP5/Dmcr rats fed an HFC diet and administrated nitric oxide (NO) synthesis inhibitor Nω- Nitro-L-arginine methyl ester hydrochloride (L-NAME) induced coronary smooth muscle cell (SMC) hypertrophy or arterial lipid deposition, and developed LV systolic dysfunction, asynergy, and replacement fibrosis. On the other hand, the SHRSP5/Dmcr rats fed an SP diet and administered L-NAME, did not present NASH pathology, or any cardiac or vascular damage [6]. These findings demonstrate that NASH aggravates the cardiovascular risk in SHRSP5/Dmcr rats. However, the intermediary factors between NASH and cardiovascular risk have not been determined so far. In our study, we focused on bile acid (BA) metabolism disorder in NASH pathology. It is well known that BAs are biosynthesized from cholesterol in the liver, excreted in the intestine via the bile duct and reabsorbed back into the liver, called enterohepatic cycle [7]. Furthermore, recent reports suggest that BAs are signaling molecules that activate nuclear receptors and membrane G protein-coupled BA receptors to regulate lipid, glucose, and energy metabolism in the liver, intestine, and adipose tissue [8]. In NAFLD/NASH patients serum BA concentration was significantly increased compared to healthy controls [9-11]. Serum BA and 7α-Hydroxy-4-cholesten-3-one levels, indicators of BA biosynthesis degree, are increased in NASH patients and correlate with hepatic histological severity [12]. In our study, we investigated whether BA metabolic disorders aggravate cardiac dysfunction in the SHRSP5/ Dmcr rat model.
Animals and feeding diets
The 9-week-old male rats of the SHRSP5/Dmcr (n=17) strain were supplied from the Disease Model Cooperative Research Association (Kyoto, Japan). The animals were maintained in a temperature (24 ± 2°C) and humidity- (55 ± 5%) controlled facility with a 12- hour light/dark cycle, and allowed to consume water and an SP diet (20.8% crude proteins, 4.8% crude lipids, 3.2% crude fibers, 5.0% crude ashes, 8.0% moisture, and 58.2% carbohydrates) ad libitum. The HFC diet (a mixture of 68% SP diet, 25% palm oil, 5.0% cholesterol, and 2.0% cholic acid) for NASH induction was obtained from the Funabashi Farm (Chiba, Japan). At 10 weeks of age the SHRSP5/Dmcr rats fed an SP or HFC diet were divided into 2 groups as follows: SHRSP5/Dmcr+SP diet+L-NAME (Non- NASH group, n=7) and SHRSP5/Dmcr+HFC diet+L-NAME (NASH group, n=10). L-NAME (N-5751, Sigma-Aldrich, Tokyo, Japan) was dissolved in Phosphate buffered saline (PBS) and administrated intraperitoneally once every two or three days from 16 until 18 weeks of age. The dosage of L-NAME was fixed at 25 mg/kg/day.
All the experimental animals were treated in strict accordance with the recommendations of the standards of the Care and Management of Laboratory Animals and Relief of Pain (2006) published by the Japanese Ministry of Environment. The protocol was approved by the Animal Experiment Committee of Okayama University (approval nos. OKU-2016535).
Dissection and blood analysis
At 18 weeks of age, blood was collected from the right carotid artery of rats that had been deprived of food overnight and anesthetized by an intraperitoneal injection of sodium pentobarbital (50 mg/ kg). Blood was centrifuged at 3,000 rpm for 30 min at 4°C and serum was collected and stored at -80°C until analysis. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and total BAs were measured with routine laboratory methods (SRL Inc., Tokyo, Japan). All rats were left to die from blood drawing, and the liver and aorta were removed and weighted. The liver weight was corrected against body weight. A part of the liver was sliced for histopathological analysis and another part was frozen for further analysis of mRNA, total cholesterol and total BA levels. The aorta was cut into 2 mm rings for evaluation of endothelial function and oil red O staining.
Histopathological analysis, oil red O and immunohistochemical staining
Liver was fixed in 10% formalin for 48 hours, embedded in paraffin and sliced for histology. Transverse sections (5 μm) were stained with the standard Masson-trichrome staining to evaluate fibrosis. All images were observed by all-in-one fluorescence microscope (BZ-X700, KEYENCE, Osaka, Japan).
The oil red O staining has been referred in previous reports [13]. Aorta was fixed with 10% formalin for 10 min. After washing with distilled water, the aorta was immersed in 60% isopropanol for 1 minute, in oil red O staining solution (O0625, Sigma-Aldrich, Tokyo, Japan) for 30 min, and in 60% isopropanol for 2 minutes.
Immunohistochemical staining of nuclear factor-kappa B (NFkB) was performed in paraffin-embedded tissue sections (3 μm). Endogenous peroxidase activity was blocked by methanol containing 0.3% H2O2. Sections were incubated with the mouse monoclonal antibody against NFkB (ab16502, Abcam plc, Cambridge, UK) at 4°C overnight and then with the Histofine Simple Stain Rat MAX PO (Nichirei Biosciences, Tokyo Japan) for 30 min.
Endothelial function
The endothelial function of each rat aorta was evaluated at the end of the experimental period. The relevant assay was performed according to previous reports [14]. The isolated aorta was cut into rings and placed in a cold Krebs-Henseleit buffer (119 mM NaCl, 4.7 mM KCl, 1.1 mM KH2PO4, 1.2 mM MgSO4, and 25 mM NaHCO3, pH 7.4). The rings were mounted at a resting tension of 1.0 g in a 5 ml organ bath (UFER UC-05A Micro-easy Magnus system, Kishimoto Medical Instruments, Kyoto, Japan) containing a warmed (37°C) and oxygenated (O2: CO2, 19: 1) Krebs-Henseleit buffer. After 60 min equilibration at this tension, the vasoconstrictor prostaglandin F2alpha (PGF2α, 0.1 μM) was added to the rings. When the constriction plateau was reached, acetylcholine (Ach, 10-10-10-3.5 M) was applied to the constricted rings to obtain concentration-response curves. The vasorelaxant effect was expressed as the percentage relaxation of the PGF2α- induced constriction. At the end of the evaluation of the endothelial function using ACh, the rings were completely dilated by addition of excess vasorelaxant (papaverine, 100 μM) to confirm mesothelium proper function.
Quantitative RT-PCR analysis
Total RNA was extracted from the liver (10 mg) and the aorta (30 mg) using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany), respectively. Extracted total RNA (1000 ng) was subjected to reverse transcription with the Primer Script RT Reagent Kit (Takara, Shiga, Japan). Quantitative PCR analysis was performed using the FastStart Essential DNA Green Master (Roche Diagnostics, Mannheim, Germany) and the Light Cycler 96 Instrument system (Roche Diagnostics, Mannheim, Germany). The specific primers for cDNAs are as follows: farnesoid X receptor (FXR; forward: 5'- CATTTACAAGCCACGGACGA-3' and reverse: 5'-CAGATTCTGCCCCAGAGGAC-3', GenBank Accession No. NM_021745.1), small heterodimer partner (SHP ; forward : 5'- CCTTGATGGCTCCCAAAACC-3' and reverse: 5'-CTGGAGGAATTCTGCCCTGA-3', GenBank Accession No. NM_057133.1), Liver X receptor (LXR; forward: 5'- TCAAAGGGTTGGGTGTTTTG-3' and reverse: 5'-TGGGTCAACAAGGCCTTCAG-3', GenBank Accession No. NM_031627.2), sterol regulatory element binding protein- 1c (SREBP-1c; forward: 5'-ATCCGGGATCCCTGTTTGTG-3' and reverse: 5'-GAAGTTTGTGGCCCATGGAT-3', GenBank Accession No. NM_001263636.1), pregnane X receptor (PXR; forward: 5'-GCCAAGGAAAGGTTCCCAAA-3' and reverse: 5'-ACCAGACCCTGGTCAGATGG-3', GenBank Accession No. NM_052980.2), constitutive androstane receptor (CAR; forward: 5'-TGTCTCTTCGTGCGGTCATC-3' and reverse: 5'-TCCTGTCTTTCCCACCCTCT-3', GenBank Accession No. NM_001270838.1), vascular cell adhesion molecule-1 (VCAM- 1; forward: 5'-GATGTCATTGCGAGGTCGTT-3' and reverse: 5'-CAGTCAGTCCAAGCAACACG-3', GenBank Accession No. NM_012889.1), intercellular adhesion molecule-1 (ICAM- 1; forward: 5'-CGGCTCCACCTCAAAGAGAA-3' and reverse: 5'-GTGCCTACCCTCCCACAACA-3', GenBank Accession No. NM_012967.1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: 5'-TCAAGAAGGTGGTGAAGCAG–3' and reverse: 5'–AGGTGGAAGAATGGGAGTTG–3', GenBank Accession No. NM_017008.4). GAPDH was used as an internal standard.
Liver total cholesterol and total bile acid analysis
Total cholesterol was extracted from the liver (10 mg) and measured by a colorimetric method (Total Cholesterol Assay Kit, CELL BIOLABS, California, USA). Total BAs were also extracted from the liver (Non-NASH group: 60 mg, NASH group: 30 mg) and measured by a colorimetric method (Total Bile Acid Assay Kit, CELL BIOLABS, California, USA). Concentrations were corrected according to liver weight.
Statistical analysis
The results obtained were statistically analyzed using Mann- Whitney U-test. The relevant data is presented as means ± standard error (SE). P-values of <0.05 were considered statistically significant.
Survival rate
The survival rate of the Non-NASH group during the experimental period was 100% (n=7/7) and of the NASH group was 70% (n=7/10). The cause of death was unknown for each individual case.
Pathological conditions and bile acid and lipid metabolism in the liver
The liver macroscopic findings revealed differences between the Non-NASH and NASH group. The NASH group showed paler and heavier livers than the Non-NASH group (Figures 1A and 1D). The Masson trichrome staining demonstrated severe fibrosis (stained by aniline blue), hepatic steatosis (observed many lipid droplets), and ballooned hepatocytes with a Mallory-Denk body formation in the NASH group (Figures 1B and 1C). In addition, hepatic function evaluated by AST and ALT was significantly worse than the Non- NASH group (Figures 2A and 2B). These findings (liver steatosis, liver fibrosis, decreased liver function, and ballooned hepatocytes with Mallory-Denk bodies) were characteristics of NASH pathology.
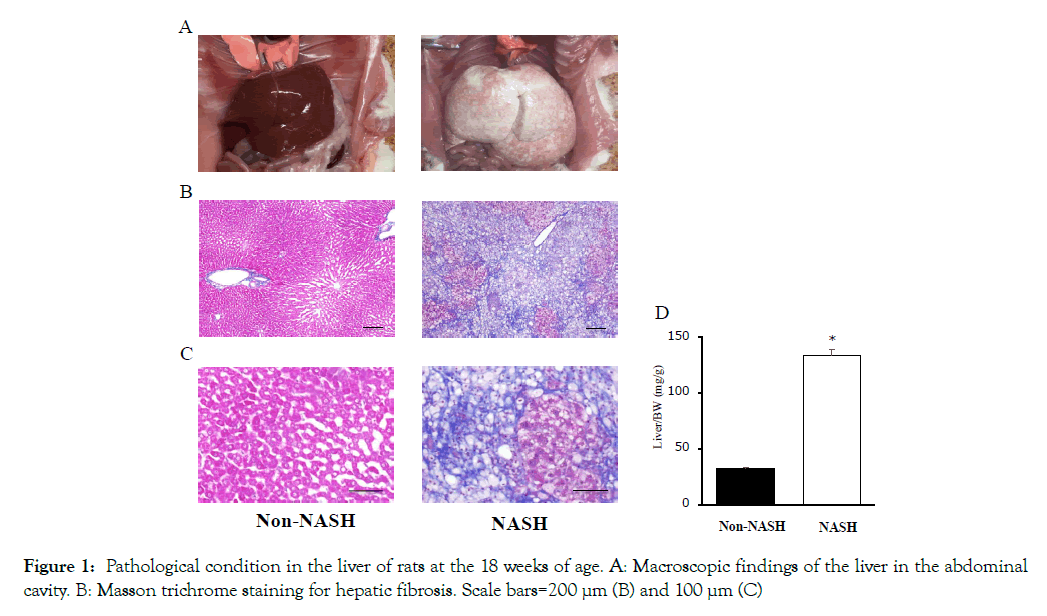
Figure 1: Pathological condition in the liver of rats at the 18 weeks of age. A: Macroscopic findings of the liver in the abdominal cavity. B: Masson trichrome staining for hepatic fibrosis. Scale bars=200 μm (B) and 100 μm (C)
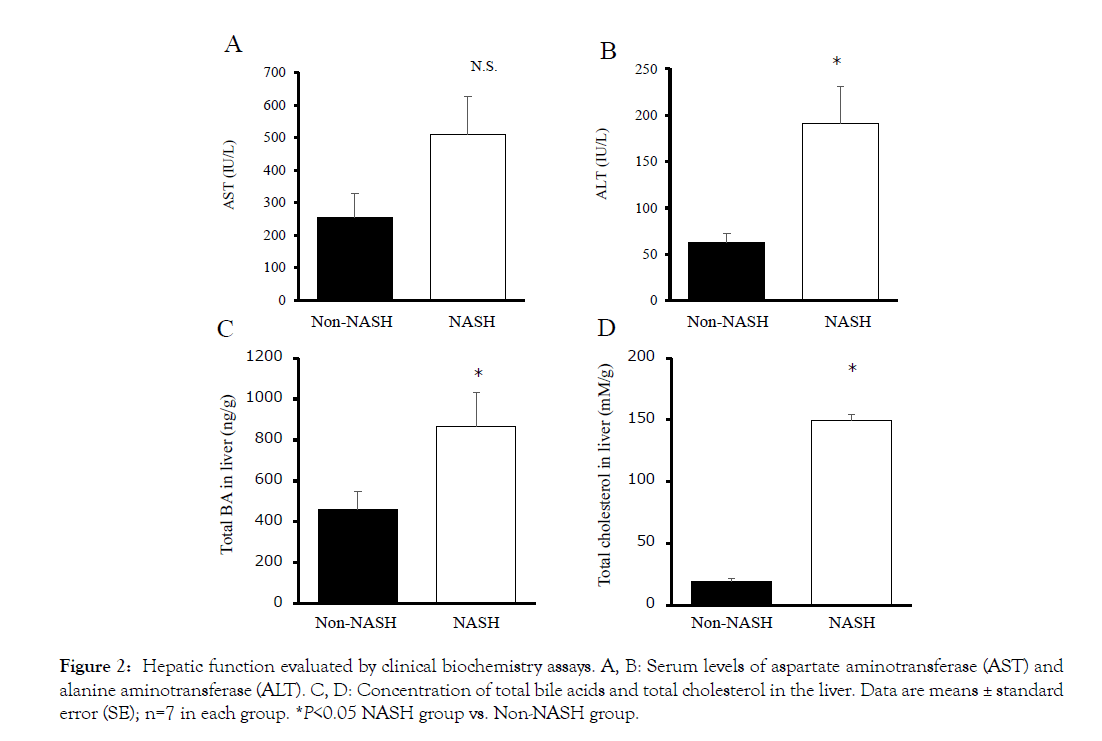
Figure 2: Hepatic function evaluated by clinical biochemistry assays. A, B: Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). C, D: Concentration of total bile acids and total cholesterol in the liver. Data are means ± standard error (SEF);i gnu=7r ein 3 e ach group. *P<0.05 NASH group vs. Non-NASH group.
The concentrations of total cholesterol and total BAs were significantly increased in the NASH group liver (Figures 2C and 2D). The SREBP-1c mRNA levels were significantly increased in the NASH group too (Figure 3A). SREBP-1c biosynthesizes triglycerides and fatty acids and is upregulated by LXR and downregulated by FXR/SHP. The LXR mRNA levels were increased and the FXR/ SHP mRNA levels were decreased compared to the Non-NASH group (Figures 3B, 3C and 3D). These differences may be related to liver steatosis in the NASH group. The mRNA levels of PXR and CAR had no significant difference between the two groups (Figures 3E and 3F). These genes belong to cytochrome P450 gene family and have a detoxifying action on BA cellular cytotoxicity based on hydrophobicity.
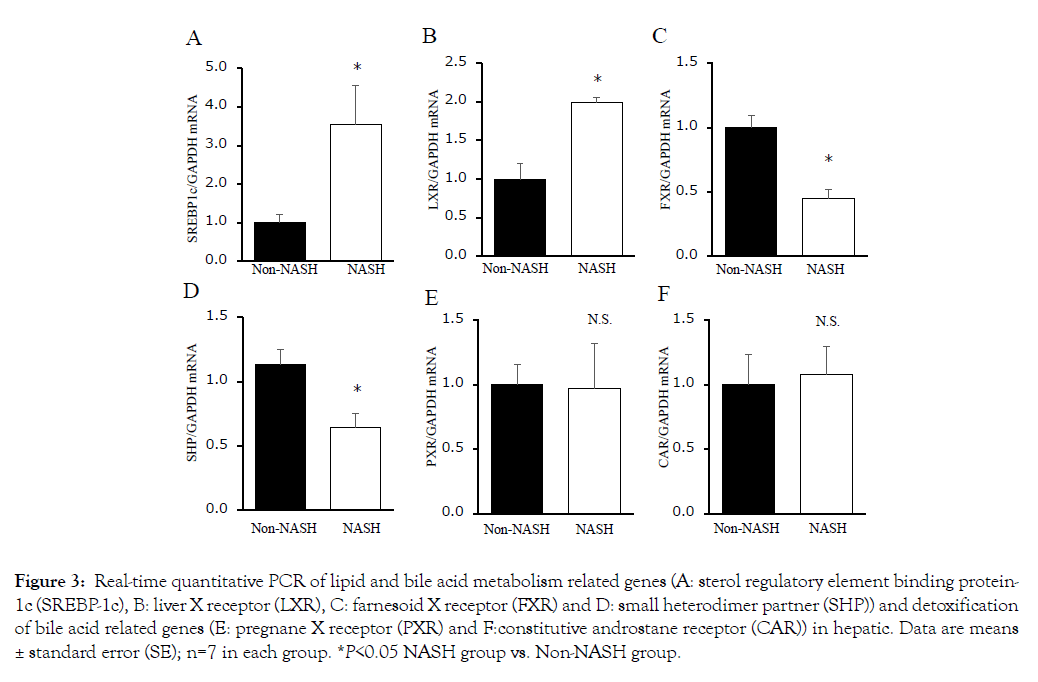
Figure 3: Real-time quantitative PCR of lipid and bile acid metabolism related genes (A: sterol regulatory element binding protein- 1c (SREBP-1c), B: liver X receptor (LXR), C: farnesoid X receptor (FXR) and D: small heterodimer partner (SHP)) and detoxification of bile acid related genes (E: pregnane X receptor (PXR) and F:constitutive androstane receptor (CAR)) in hepatic. Data are means ± standard error (SE); n=7 in each group. *P<0.05 NASH group vs. Non-NASH group.
Endothelium and atherogenesis
The mRNA levels of cell adhesion molecules (ICAM-1 and VCAM-1) in coronary artery endothelial cells were significantly upregulated in the NASH group (Figures 4A and 4B). The immunohistochemical staining of NFkB in the NASH group demonstrated strong positivity in the endothelial cells compared to the Non-NASH group (Figure 4C). L-NAME, which induces NO synthetase inhibition, interferes in vasodilatation via an acetylcholine. Despite L-NAME administration to both groups, the endothelial function in the NASH group was obviously impaired compared to the Non-NASH group. The dilatation percentage of maximum contraction was 80% in the NASH group and 60% in the Non-NASH group, even at acetylcholine concentration of 10- 3.5M (Figure 5). These results indicate that increasing expression of cell adhesion molecules and endothelial dysfunction may be caused not only by L-NAME, but also by NASH pathology. In addition, many lipid depositions in the abdominal aorta were observed in the NASH group (Figure 6). In contrast, the Non-NASH group had fewer and less obvious lipid deposits than the NASH group. In the NASH group, many lipid deposits in the abdominal aorta were observed in every rat.
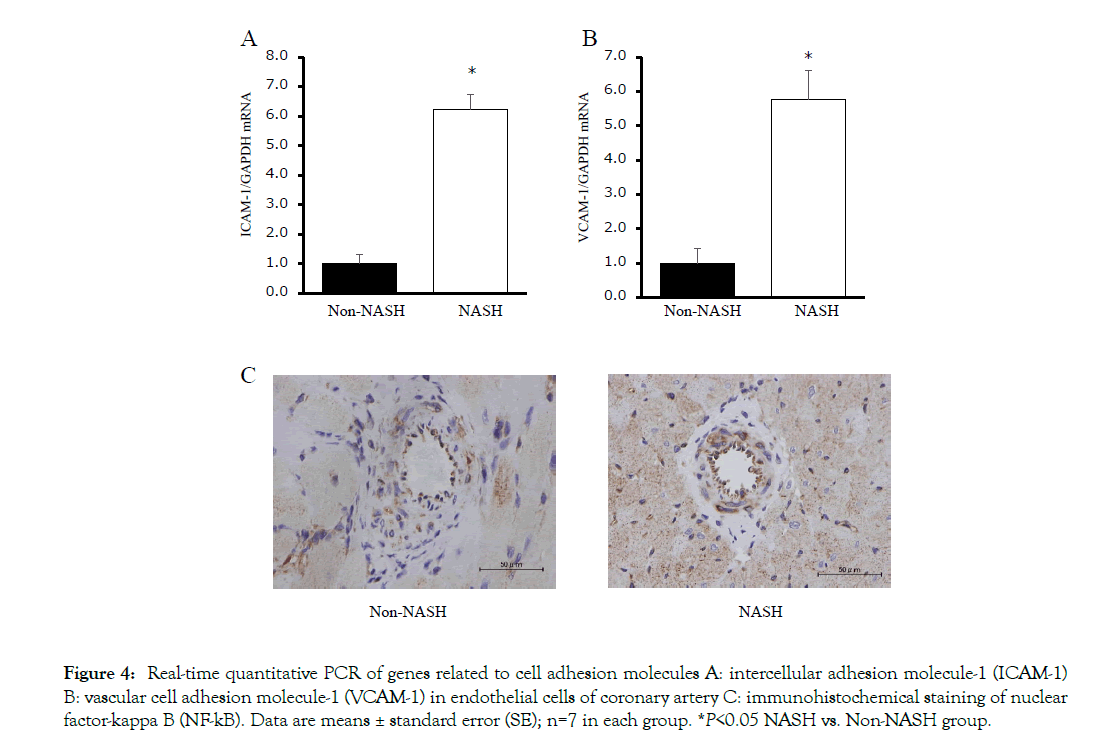
Figure 4: Real-time quantitative PCR of genes related to cell adhesion molecules A: intercellular adhesion molecule-1 (ICAM-1) B: vascular cell adhesion molecule-1 (VCAM-1) in endothelial cells of coronary artery C: immunohistochemical staining of nuclear factor-kappa B (NF-kB). Data are means ± standard error (SE); n=7 in each group. *P<0.05 NASH vs. Non-NASH group.
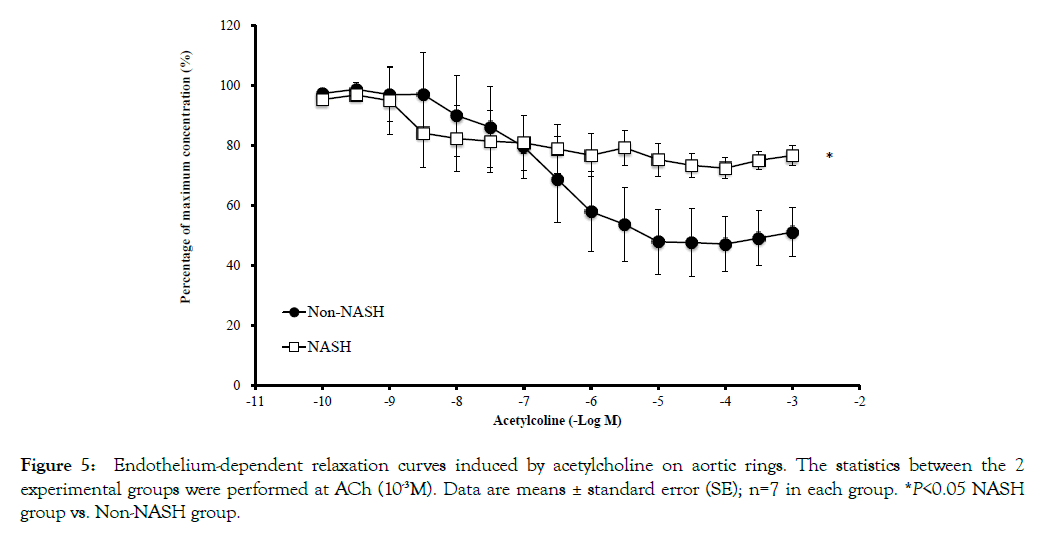
Figure 5: Endothelium-dependent relaxation curves induced by acetylcholine on aortic rings. The statistics between the 2 experimental groups were performed at ACh (10-3M). Data are means ± standard error (SE); n=7 in each group. *P<0.05 NASH group vs. Non-NASH group.
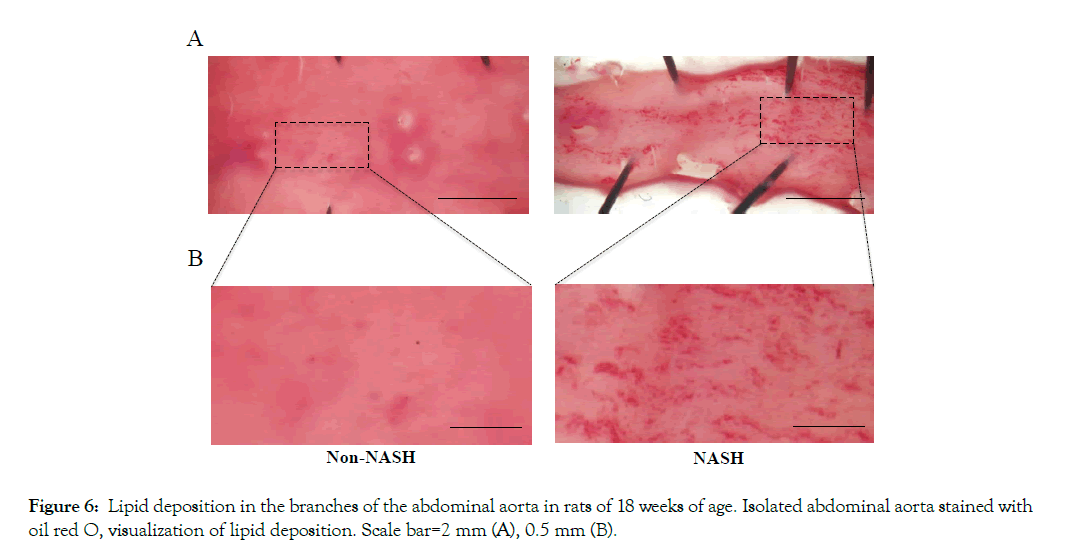
Figure 6: Lipid deposition in the branches of the abdominal aorta in rats of 18 weeks of age. Isolated abdominal aorta stained with oil red O, visualization of lipid deposition. Scale bar=2 mm (A), 0.5 mm (B).
Clinical biochemistry data
In the NASH group, which fed an HFC diet included cholesterol, serum total cholesterol, LDL-cholesterol and LDL-/HDL-cholesterol ratio were remarkably increased (Table 1). The serum total BA levels in the NASH group were also significantly increased compared to the Non-NASH group. However, triglycerides were decreased, and HDL-cholesterol had no significant difference.
| Parameters | Non-NASH group | NASH group |
|---|---|---|
| Total bile acid (mg/dL) | 0.14 ± 0.01 | 0.96 ± 0.24* |
| Total cholesterol (mg/dL) | 97.2 ± 8.6 | 429.5 ± 84.3* |
| HDL-cholesterol (mg/dL) | 31.5 ± 2.7 | 28.3 ± 2.3 |
| LDL-cholesterol (mg/dL) | 14.3 ± 1.5 | 136.3 ± 18.1* |
| Triglyceride (mg/dL) | 52.3 ± 8.4 | 28.0 ± 2.7* |
| LDL-/HDL- cholesterol ratio | 0.47 ± 0.08 | 4.81 ± 0.48* |
*P<0.05 NASH vs. the Non-NASH group.
Table 1: Bile acid and lipid metabolism of blood analysis in rats at 18 weeks of age n=7 in each group.
The SHRSP5/Dmcr rat model, which has congenital hypertension, induces NASH, lipid deposition at the mesenterial artery, and hyperlipidemia due to HFC diet [15,16]. In addition, we previously reported that the SHRSP5/Dmcr rat model fed an HFC diet and administered L-NAME, demonstrated characteristics similar of those of the ischemic heart disease; (1) LV systolic dysfunction associated with asynergy, (2) replacement fibrosis caused by shedding of cardiomyocytes, and (3) coronary SMCs hypertrophy and arterial lipid deposition caused by endothelial dysfunction [6]. However, the intermediary factors between NASH and ischemic heart disease were not identified. Ischemic heart disease is caused by obstruction of the coronary artery, and it is usually aggravated by atherosclerosis, a buildup of plaques inside the artery walls. In the present study, we observed increased serum total BA levels (Table 1), endothelial dysfunction in aorta which is the first step of atherosclerosis (Figure 5) and high expression levels of cell adhesion molecules (ICAM-1 and VCAM-1) in coronary artery (Figures 4A and 4B). Recently, some reports suggested that serum BA levels are increased in NASH patients [9-11], and BAs are one of the risk factors in atherosclerosis [17]. These evidences show that serum bile acids may be an intermediary factor between NASH and cardiovascular risk. We discuss the relationship of “NASH and BAs” and “BAs and atherosclerosis” as shown in Figure 7.
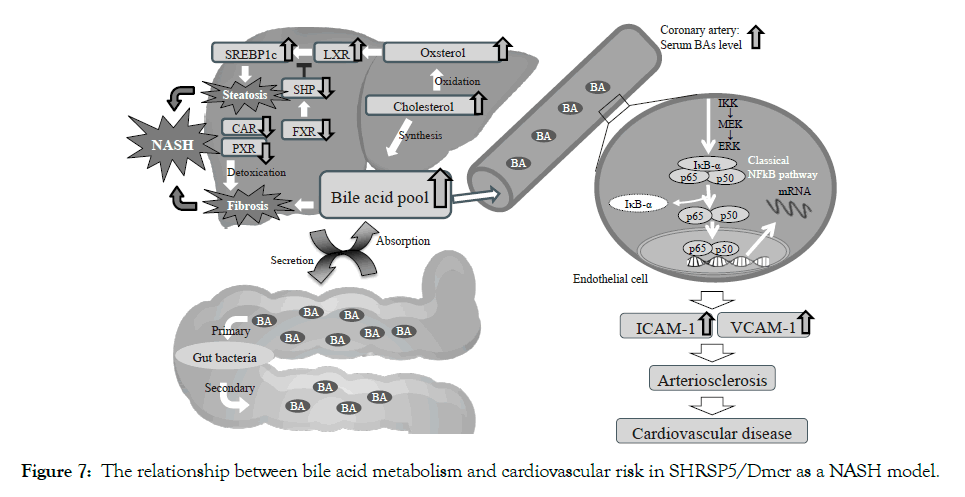
Figure 7: The relationship between bile acid metabolism and cardiovascular risk in SHRSP5/Dmcr as a NASH model.
NASH and bile acids metabolism
The NASH pathology is developed by multiple parallel hits: oxidative stress, decrease of anti-oxidants, ATP depletion, cytokine formation, ER stress, adipokines, bacterial endotoxins, etc. [18,19]. Recent reports suggest that the concentration of total BAs was increased in liver tissue and plasma of NASH patients compared to NAFLD patients [10]. BAs are well known for their role in lipid absorption. BAs exhibit great surface activity related to their amphipathic nature and formation of small aggregates or micelles for lipid transport [20]. However, several BAs, for example deoxycholic acid (DCA) and lithocholic acid (LCA), have higher cellular cytotoxicity compared to others, as cholic acid (CA), chenodeoxycholic acid (CDCA), and ursodeoxycholic acid (UDCA) [21]. Although we could not analyze BA isotypes in this study, total BA liver concentration was significantly increased in the NASH group compared to the Non-NASH group (Figure 2C). Jia et al. also reported that total BA liver concentration was increased by an HFC diet for 2 weeks in SHRSP5/Dmcr rats [22]. In NASH patients, BA liver concentration was increased and significantly associated with histologically proved steatosis, inflammation and fibrosis [23]. It is considered that BA accumulation aggravated liver steatosis in SHRSP5/Dmcr rats.
Despite of the fact that total BA concentration was obviously different between the two groups, the mRNA levels of PXR and CAR were equivalent (Figures 3E and 3F). These results were different in previous reports [24]. PXR and CAR are nuclear receptors belonging to cytochrome P450 family and having the role of detoxifiers against various harmful substances and medicines [25]. Naito et al. suggested that the mRNA levels of PXR and CAR were decreased in the SHRSP5/Dmcr rats fed an HFC diet compared to those in rats fed an SP diet [24]. Roth et al. reported that SREBP-1c was inhibited by PXR and CAR in rodents [26]. The mRNA levels of PXR and CAR in SHRSP5/Dmcr rats were decreased compared to Wister Kyoto (WKY) rats, used as controls of SHRSP5/Dmcr (Data not shown). This phenomenon may be characteristic of the SHRSP5/Dmcr model. Therefore, BA accumulation and downregulation of detoxicate activity (PXR and CAR) may induce hepatocyte damage, inflammation and fibrosis in SHRSP5/Dmcr rats (Figure 7).
NASH have characteristic not only fibrosis, but also steatosis. LXR is an oxysterol receptor derived from cholesterol catabolism, which aggravates lipid accumulation by activating the lipid synthesis transcription factor (SREBP-1c) (Figure 7). In the present study, cholesterol and mRNA levels of LXR and SREBP-1c were increased in the NASH group (Figures 2D, 3A and 3B). The FXR mRNA levels were decreased in NASH pathology SHRSP5/Dmcr rats (Figure 3C). It is considered that FXR is involved in the formation of fatty liver via regulation of homeostasis and SREBP-1c activity via SHP [27]. In clinical studies, NAFLD/NASH patients showed decreased FXR protein expression [28]. The activation of SREBP- 1c may be involved not only in the LXR, but also in the FXR/ SHP pathway. It is considered that high BA concentrations may be generated by cholesterol excess in NAFLD/NASH patients, because BAs are synthesized from cholesterol. The BA metabolism in NAFLD/NASH patients has attracted much attention in recent years and several therapeutic agents such as UDCA and obeticholic acid (OCA) has been developed [29]. Leuschner et al. gave to 185 non-cirrhotic NASH patients UDCA (23-28 mg/kg daily) or placebo for 18 months. The authors reported a positive drug effect only on lobular inflammation and not on fibrosis or on laboratory data [30]. Neuschwander-Tetri BA et al. reported that OCA (25 mg daily for 72 weeks) improved the histology in 45% of the non-cirrhotic NASH patients, compared to 21% of the placebo patients [31]. These BA receptors such as FXR and G-protein-coupled bile acid receptor (Gpbar1, TGR5) ameliorate BA biosynthesis, energy, lipid and glucose-homeostasis [32]. Therefore, the understanding of the contribution of BA dysregulation for the pathogenesis and progression of NAFLD/NASH is very important.
Bile acids as a risk factor of atherosclerosis
In patients with NASH chronic inflammation evolves into arteriosclerosis, thereby increasing the cardiovascular risk during NAFLD progression in a larger extent than in patients with simple fatty liver [33]. In fact, serum levels of chronic inflammation liver markers, for example C-reactive protein, interleukin-6, tumor necrosis factor-alpha, plasminogen activator inhibitor-1 and fibrinogen, are increased in NAFLD/NASH patients [34]. Obesity, insulin resistance, cholesterol and oxidative stress are also atherosclerosis risk factors. However, the SHRSP5/Dmcr rat model does not show obesity and insulin resistance [5]. Serum cholesterol level is the common risk factor of cardiovascular disease. In fact, serum LDL cholesterol levels are obviously increased in the NASH group (Table 1). Atorvastatin significantly decrease cardiovascular disease risk in NASH patients who do not present obesity and insulin resistance [35].
This finding suggests that LDL cholesterol in NASH patients is deeply involved in atherosclerosis; LDL cholesterol is one of the factors that promoted arteriosclerosis in our study. However, WKY rats fed an HFC diet showing simple fatty liver and high LDL cholesterol levels did not demonstrate atherosclerosis at all. We consider that not only LDL cholesterol, but also BAs can act as intermediary factors between NASH and cardiovascular risk. In in vitro studies, BAs upregulated ICAM-1 and VCAM-1 expression via the classical NF-kB pathway in the endothelial cell (Figure 7). The mRNA levels of VCAM-1, Interleukin-4 and Tumor necrosis factor a were increased in a concentration dependent manner in the placental cytotrophoblast cell via activation of the PI3K/ NF-kB pathway [36]. In addition, several reports suggested that serum BAs upregulated ICAM-1 and VCAM-1 expression via the classical NE-kB pathway [37,38]. ICAM-1 and VCAM-1 are well-known cell adhesion molecules that accelerate the developing of atherosclerosis. In the present study, serum total BA levels (Table 1), as well as protein levels of NF-kB in the coronary artery endothelial cell and mRNA levels of ICAM-1 and VCAM-1 (Figure 4) were significantly increased. Charach et al. reported that patients who presented low BA excretion after daily intake of cholesterol (500 mg) for 4 consecutive days demonstrated higher mortality and cardiovascular disease rates at a 20-year follow up [39]. Jovanovich et al. reported that high serum DCA levels correlated significantly with developing vascular calcification for 9 months in chronic kidney disease patients [40]. On the other hand, several reports mention that serum BAs protect cardiac function via FXR and/ or TGR5 activation [41,42]. There are various isotypes of BAs, for example CA, DCA, CDCA, LCA and UDCA. The cellular cytotoxicity and strength activation of these receptors characterize each BA isotype. It might be important to measure not only total bile acid levels, but also each BA isotype levels.
The SHRSP5/Dmcr rat model, which includes rats with congenital hypertension, induces NASH and cardiovascular disease, such as coronary SMC hypertrophy, arterial lipid deposition, LV systolic dysfunction, asynergy, and replacement fibrosis after administration of an HFC diet and L-NAME. We demonstrated that hepatic BA accumulation aggravates steatosis and fibrosis in NASH, and serum BA activates cell adhesion molecules in the coronary artery via the classical pathway of NFkB. The BA metabolism disorder may be an intermediary factor between NASH and increased cardiovascular risk.
This work was supported by grants from the Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (B), Grant Number 15K19178 and Grant-in-Aid for Scientific Research (C), Grant Number 18K10993 to S. Watanabe.
Conflict of interest
Shota Kumazaki, Mayu Nakamura, Shun Sasaki, Rina Tagashira, Nozomi Maruyama, Ikumi Sato, Shusei Yamamoto, Shang Ran, Shinichi Usui, Ryoko Shinohata, Takashi Ohtsuki, Satoshi Hirohata, Kazuya Kitamori, Mari Mori, Yukio Yamori and Shogo Watanabe declare no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human subject, and all experimental animals were treated in strict accordance with the recommendations of the standards of the Care and Management of Laboratory Animals and Relief of Pain published by the Japanese Ministry of Environment in 2006. The protocol was approved by the Animal Experiment Committee of Okayama University (Approval nos. OKU-2016535).
Citation: Kumazaki S, Nakamura M, Sasaki S, Tagashira R, Maruyama N, Sato I, et al. (2019) Bile Acid Metabolism is an Intermediary Factor between Non-Alcoholic Steatohepatitis and Ischemic Heart Disease in SHRSP5/Dmcr Rats. J Nutri Food Sci. 9: 763. doi: 10.35248/2155-9600.19.9.763
Received: 12-Sep-2019 Accepted: 10-Oct-2019 Published: 18-Oct-2019 , DOI: 10.35248/2155-9600.19.9.1000763
Copyright: © 2019 Kumazaki S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.