Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2014) Volume 5, Issue 1
Malaria, transmitted by mosquitoes of the genus Anopheles, is the most important parasitic disease worldwide. It affects 40% of the global population mostly within the tropical world. Yearly, over one million children under the age of five die in Africa as a result of malaria. The genus Turraea belongs to the family Meliaceae. Meliaceae is characterized by the presence of tetranortriterpenoids (limonoids), a group of compounds that exhibit a wide variety of biological properties including anti-insect, anti-protozoa, anti-bacterial and anti-fungal activities. In the present study, the root bark of T. abyssinica and T. cornucopia were extracted with methanol and partitioned between water and chloroform. The activity of crude methanol and chloroform extracts was tested against larvae and adults of Anopheles gambiae sensu stricto (Diptera: Culicidae). As larvicides, the methanol extract of T. cornucopia was the most active (LD50 202 ppm). The chloroform extract of T. cornucopia was the most active as an adulticide (LD50 302.1 ppm). Partitioning of the methanolic extracts between chloroform and water, followed by silica gel chromatography of the organic extract gave limonoids-rich fractions that had larvicidal activity higher than those of the corresponding crude extracts. Column chromatography of the chloroform fraction followed by semi-preparative HPLC yielded 1α-12α-diacetoxy-1,2-dihydro-7-deacetyl-3β-7α-dihydroxyazadiron,12α-acetoxy-7-deacetylazadiron and mzikonone. The structures of these compounds were elucidated using spectroscopic methods (IR UV, MS, 1H-, 13C- NMR, gradient COSY, and gradient HMBC experiments). Stereo chemical assignments were made by gradient NOE spectroscopy. These plants offer a significant potential for mosquito control as larvicides and adulticides.
<Keywords: Turraea cornucopia, Turraea abyssinica Meliaceae, Limonoids, Anopheles gambiae, Assays
Malaria is by far the most devastating and deadly parasitic disease in the world. It is a public health problem in more than 90 countries inhabited by over 2.4 billion people (approximately 40% of the world’s population). The disease is estimated to cause up to 500 million clinical cases and 2.7 million deaths each year [1]. Of the people infected, Africa accounts for over 90% of the reported cases. Experts foresee as much as 20% annual increase in Africa’s rate of malaria related illnesses and deaths [1]. The mortality rate is high in children under five years and pregnant women [1].
The cost of malaria when viewed in economic terms is enormous. In most African countries, over a quarter of a family’s income goes towards the cost of malaria treatment [1]. This is in addition to the cost of prevention or the opportunity cost of labour lost during illness. Each bout of malaria causes its victim to forego an average of twelve days productive output. People are more at risk during the warm and rainy season. This is usually when there is most agricultural activity. Malaria and fear of malaria prevents investment and tourism in new regions. The global effects of malaria threaten public health and productivity on a broad scale and impede economic progress in many countries [1].
Malaria, which had been effectively controlled in many parts of the world through the use of antimalarials like quinine and chloroquine, insecticides like p,p-dichloro-2,2-diphenyl-1,1,1-trichloroethane (DDT) and pyrethrins among others, is undergoing resurgence [2] Most epidemics have been linked to climatic change, [3-6] and drug and insecticide resistance [7]. Demographic changes have resulted in more people moving into already densely populated areas thereby increasing transmission. In areas where development projects like agro forestry, irrigation projects, road construction and mining have been introduced, new breeding sites are created [5]. In many regions malaria control programs have deteriorated or been abandoned due to high costs of sustaining them. Renewed efforts in malaria control are required.
Meliaceae is a family of woody tropical plants comprising approximately 50 genera and a total of 500-550 species. Chemically, the family Meliaceae is characterized by its biosynthesis of limonoids, which are modified triterpenes with or derived from a precursor with a 4,4,8-trimethyl-17-furanyl steroid skeleton [8,9]. Over 300 limonoids have been isolated to date, and they are more diverse and abundant in Meliaceae than in any other family.
Traditionally in certain parts of Africa, some Meliaceae species are used for treatment of fevers and malaria [10]. In west and east Africa, the Meliaceae species A. indica is used for treatment of malaria [11,12]. In tropical America, members of the Meliaceae family, Cedrela odorata, Carapa guianensis and Swietenia mahagoni have been used in traditional medicine for the treatment of fevers, a characteristic symptom of malaria [13].
Melia azedarach Linn. (China berry or Persian lilac tree) has long been recognized as a medicinal and insecticidal plant [14]. One of the constituents from M. azedarach is azadirachtin, a highly potent limonoid insect antifeedant and ecdysis inhibitor [15]. The roots of Chukrasia tabularis (Meliaceae) have been reported to be active against chloroquine resistant strains of P. falciparum [9].
Extracts of the leaves of A. indica, Cedrela salvadorensis and the wood of C. odorata and Dysoxylum fraseranum are reported to have high activity against chloroquine sensitive P. falciparum [13].
This work is therefore, undertaken to investigate the potential of plants belonging to the family Meliaceae, known from previous research to possess anti-arthropod properties, in the control of malaria vectors.
General experimental procedures
All recyclable glassware used was washed in hot water and soap and rinsed with distilled acetone. The glassware was then dried at 100°C for one hour. All the solvents and reagents used were obtained from Aldrich Chemical Co. Ltd, England and Merck, Germany.
Plant materials
T. abyssinica was collected from Kijabe while T. cornucopia was collected from Ngong forest both in Rift Valley Province. They were identified by Mr S.G. Mathenge of the Botany Department, University of Nairobi. Voucher specimen number 2003/203 for T. cornucopia and 2003/211 for T. abyssinica have been deposited in the Herbarium of that Department.
Mosquito larvae and adults
Anopheles gambiae s.s larvae were reared under standard laboratory conditions. This strain of mosquitoes originates from Mbita, and has been reared under laboratory conditions in the ICIPE mosquito insectary. Eggs were allowed to emerge in plastic containers filled with distilled water, and were transferred to larger pans at densities of 200- 300 at L2 (second larvae instar) stage. Water temperature was kept constant by heating the insectary and varied only slightly between 28- 30°C. Larvae were fed on Tetramin fish food (Tetr Werke Germany) and were used for experimental purposes upon reaching the late L3 or early L4 developmental stage.
The adults have been reared under laboratory conditions in the ICIPE mosquito insectary. Room temperature was kept constant between 26-28°C. 2-5 Days old mosquito adults were used for experimental purposes.
Extraction procedures
The air-dried root bark of T. cornucopia was powdered (960 g) and extracted in the dark by soaking for three weeks with methanol. The methanol extract was concentrated in vacuo to yield viscous red oils (94 g). The methanol filtrate was partitioned between water and chloroform (1:2.5 (three times). The combined organic layer was then concentrated to dryness (6.6 g). The chloroform extract (6g) was column chromatographed on silica gel (81×4.5 cm; 230-400 mesh glass columns) using hexane: ethyl acetate gradient (100:0 to 0:100). Separation was monitored by thin layer chromatography (TLC). The TLC plates were developed with hexane-ethyl acetate (2:1), sprayed with Ehrlich’s reagent (2% 4-dimethylaminobenzaldehyde in ethanol) and developed in a hydrogen chloride gas chamber. Typical pink/ reddish colored spots were obtained for limonoids-rich fractions eluted at 50-100% ethyl acetate. The non limonoid fractions were pooled into one fraction, TC F1 (1.2 g). Other fractions were TC F2 (1.13 g), TC F3 (464 mg) TC F4 (723 mg), TC F5 (605 mg) and TC F6 (578 mg).
The active fractions, TC F4 (150 mg) and TC F5 (105 mg) were rechromatographed on another silica gel column (50×2 cm; 230-400 mesh) using 30% acetone-hexane eluent to yield fractions TC F41 (52 mg) and TC F51 (44 mg) which were further purified by preparative HPLC (Ultrasphere ODS reverse phase column (250×10 mm, 5 μm particle size). The elution was carried out at a flow rate of 2.0 ml per minute under isocratic conditions using acetonitrile-water eluent (60:40). The compounds were detected by UV absorption at 215 nm. The HPLC fractions were evaporated to dryness in vacuo. Three pure compounds 1 (5 mg), 2 (4 mg) and 3 (8 mg) were obtained. The purity of the isolated compounds was confirmed by analytical HPLC (Ultrasphere ODS reverse phase column (250×4.6 mm, 5 μm particle size).
Biological activity tests
Larvicidal assays: Larvicidal activities of both the methanol and chloroform extracts were subjected to standardized WHO bioassays for larvicidal activity. The stock solution was made by dissolving 500 mg of crude methanol extract in 1 ml of DMSO and 9 ml of distilled water to obtain a concentration of 50 mg/ml. Subsequent lower concentrations (1000, 750, 500, 250, 100 and 50) ppm were made by diluting the stock solution 50, 67, 100, 200, 500 and 1000 fold respectively by making to 100 ml with distilled water in a 250 ml beaker. For the chloroform extract, 500 mg of the extract was dissolved in 10 ml of acetone and the same procedure repeated as for the methanol extract. Twenty late L3 or young L4 instar larvae were used. Mortality was observed after 24 hours. In concentrations where mortality was delayed for more than 24 hours, mortality was assessed every 24 hours up to emergence of the adults or death of the last larva or pupa. During the experiment, larvae were fed with Tetramin® fish food. From the results, the probit mortality/log dose regression, and hence the LD50 was computed for the respective test materials.
Adulticidal assays: Adulticidal assays were conducted with the same series of doses as those for larvicidal bioassay. The test material was applied to filter paper (Whatman No.1) 12×15 cm. The papers were air dried for one hour and then inserted into sterile petri dishes. The lids of the petri dishes were perforated for ventilation to avoid suffocation of the mosquitoes. Groups of 20 non-blood-fed female A. gambiae, 2 to 5 days old, were placed in each petri dish, exposed to the treated paper for twenty minutes and returned to holding cages (12×12 cm) for the determination of the 24 hour dosage/mortality relationship. The dosage/mortality curve was replicated on three separately reared batches to allow for inter-batch variability. From the results, the probit mortality/log dose regression, and hence the LD50 was computed for the respective test materials.
Data analysis
The larvicidal and adulticidal effects of different extracts with varying concentrations were tested on A. gambiae larvae and adult against a standard.
Repeated-measures analysis of variances (ANOVA) was applied so as to test the effects of different dosages (DOSE), different extracts (CHEM) and their interaction (DOSE* CHEM) on the response variable (PERCENT MORTALITY) on A. gambiae larvae and adults.
Abbot’s formula was used to adjust mortality in treatment with mortality in control, that is
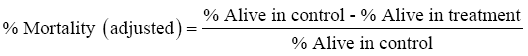
The adjusted mortality was transformed to a scale to fit a general linear model (GLM)
Turkey test was used for comparison of means. Log10 probit analysis was used to compute the LD50 values.
Statistical analysis system (SAS) version 8.2 was used.
Structure elucidation experiments
Chromatography: Column chromatography was performed on silica gel 60 (0.40-0.063 mm, 230-400 Mesh ASTM) and thin layer chromatography (TLC) on precoated silica gel 60F254 plates (0.2 mm thickness, Merck). Semi- preparative HPLC work was done on Beckman Ultrasphere ODS column, 250×10 mm, on Varian 5000 liquid Chromatograph. Analytical HPLC was done on Beckman Ultrasphere ODS column, 250×4.6 mm, on Beckman System Gold Chromatograph
Ultra violet spectroscopy: The ultra violet spectra of purified compounds was determined using an online diode array detector (Module 168) on a Beckman HPLC (System Gold), UVλmax was determined in acetonitrile.
Infra-Red (1R) spectroscopy: The IR spectra of the compounds were recorded using Shimadzu Fourier Transform infrared spectrophotometer (FT-IR-8400). The samples were prepared in KBr discs. The spectrum was recorded after background correction in the range 4000-400 cm-1.
Nuclear Magnetic Resonance (NMR) Experiments: NMR data was recorded at room temperature on Bruker Avance 500 (500 MHz). The spectra were recorded in CDCl3 solvent. Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane (TMS).
Melting point: Melting points (Mpt) were determined using a Yanaco micro melting point apparatus model MP500D and the values were uncorrected.
Larvicidal bioassays for the methanol and chloroform extracts
The crude extracts displayed potent larvicidal activity. The results of the activities for the extracts are given in Figure 1. The results show that the mortality of larvae increased as doses of the extracts were increased. High mortality (>90% mortality) values were observed within 24 hours of treatment with 750-1000 ppm dose of all the methanol extracts. It was also observed that the methanol extracts of T. abyssinica and T. cornucopia had the same level activity as azadirachtin at this dose. The activity of methanol extracts at 50 and 100 ppm were not significantly different at 95% confidence interval for T. abyssinica. For T. cornucopia, the activity of the methanol extracts at 500, 750 and 1000 ppm were not significantly different at 95% confidence level. At the 250 ppm dose, T. cornucopia extract was the most active giving a mortality value of 58%. At 100 ppm dose the activities of both T. abyssinica and T. abyssinica extracts were not significantly different. The methanol extracts were more active than the standard azadiractin at the 50 ppm dose.
For the methanol extract, T. cornucopia displayed an LD50 value of 202 ppm while T. abyssinica had an LD50 value of 265 ppm. For the chloroform extract, T. abyssinica presented an LD50 value of 350 ppm while T. cornucopia showed LD50 value of 281 ppm. Azadirachtin had an LD50 value of 145 ppm compound.
The differences in activity between azadirachtin and the crude extracts at the lower doses could be attributed to the fact that the total blend of compounds in the crude extracts may have synergistic effect on the level of activity as opposed to the pure azadirachtin.
The chloroform extracts showed the same activity at the highest dose, 1000 ppm. At 750 ppm dose, after 24 hours larval mortality was 100% for all the chloroform extracts, except for the T. abyssinica extract which had a mortality of 97%. At 500 ppm dose, T. abyssinica extract exhibited an activity of 60% while T. cornucopia extract had an activity of 95% that was not significantly different from that of azadirachtin. At 250 ppm dose, T. cornucopia extract was the most active giving mortality value of 35%. At 100 ppm dose, T. cornucopia extract exhibited no activity while T. abyssinica extract had an activity of 8%. There was no significant difference between the larvicidal activities of the extracts at the 50 ppm dose.
Larval mortality was dose dependent across the dose range tested for both the methanol and chloroform extracts. The methanol extract showed enhanced larvicidal activity as compared to the chloroform extract. The methanol extract showed some delayed larval mortality. At 100 ppm, mortality was low (15%) in 24 hours, but increased on prolonged exposure of the larvae to the treatment, up to 91% at day seven. A dose of 50 ppm had larvicidal activity of 91% on the eighth day. The surviving larvae moulted into pupae that emerged into adults.
The chloroform extracts showed a clear pattern of dose dependent effects. After 24 hours mortality was 100% at 1000 ppm. This reduced to 60% at 500 ppm and only 1% at 50 ppm. Seven days after treatment, mortality increased considerably to 49% for the 50 ppm dose.
Fractionations of the chloroform soluble fraction of T. abyssinica yielded four limonoids-rich fractions. The non-limonoid fractions were combined to make one fraction TAF1 that induced the least activity with an LD50 of 293.3 ppm. Fraction TAF4 eluted at 85% ethyl acetate elicited the highest mortality with an LD50 185.4 ppm. The larvicidal activities of all the fractions increased on prolonged exposure of larvae to the treatment.
At the 50 ppm dose of the methanolic extract, larval mortality was only 1% after 24 hours of exposure. Mortality increased to 100% after 7 days of exposure of larvae to the treatment at the same dose. 1000 and 750 ppm doses showed larvicidal activity of 100% after 24 hours. Larval mortality at 500 and 250 ppm dose was 96% and 58%, respectively after 24 hours.
Larvicidal activity of the chloroform soluble fractions was comparable to the methanol extracts at higher doses but showed delayed larval mortality at lower doses. A dose of 50 ppm had little larvicidal activity which increased to 45% on the tenth day. The surviving larvae moulted into pupae that emerged into adults.
Fractionation of the chloroform soluble fraction of T. cornucopia yielded five limonoids-rich fractions. The non-limonoid fractions were combined to make one fraction TCFI that was the least active with LD50 value of 425.9 ppm. Fraction TCF5 eluted at 70% ethyl acetate induced the highest mortality (LD50 of 56.6 ppm).
The differences of the bioactivities could be attributed to the fact that plant species do not always have an identical chemical composition and quantities, as the synthesis of bioactive compounds is regulated by different stress factors, such as soil temperature, soil type, pressure and overall climate of the area.
Adulticidal activity
The adulticidal activity for all the extracts was dose dependent. The chloroform extracts exhibited higher activity than the corresponding methanol extracts. As an adulticide, the chloroform extract was most potent, with an LD50 value of 302.1 ppm which was lower than that of azadirachtin at 552.2 ppm. The methanol extract was the least active as an adulticide with an LD50 value of 1569 ppm. At 100 ppm dose, there was no significant difference between azadirachtin and the methanol and chloroform extracts of T. cornucopia at 95% confidence interval.
Structure elucidation for isolated compounds
In light of the larvicidal and adulticidal activities of these extracts, the most active fractions were analysed to identify the individual constituents in order to find out which ones are responsible for the activity.
The compounds 1-3 were obtained from semi-preparative HPLC of the respective silica gel column chromatography fractions of T. cornucopia. All were obtained as white powders. The purity of the isolates was confirmed by analytical HPLC. The compounds were characterized by analysis of their NMR, IR, UV and MS spectra data.
Compound 1: High resolution mass spectrometry of compound 1 showed molecular ion peak at 454 (Calculated value of 454.6052). Fragment ion peaks were observed at m/z 394 [M-CH3COOH]+ and 376 [M-CH3COOH-H2O]+ due to the loss of acetic acid and water. The molecular formula of C28H38O5 was deduced from the mass spectrum in conjugation with the 1H and 13C NMR spectrum. The infrared spectrum (KBr) indicated the presence of a hydroxyl (3423 cm-1), an ester (1737 cm-1), a ketone (1702 cm-1), C-O (1248 cm-1) and an olefinic double bond (1652 cm-1). The presence of the ketone and ester was further substantiated by 13C NMR absorptions at δ 216.9 and δ 170.8 respectively. The limonoid β-substituted furan ring proton occurred at δ 7.34, 7.21 and 6.26 in the 500 1H NMR spectrum. A proton resonance at δ 5.68 that corresponds to a methine carbon resonance at δ 122.6 in the HMQC spectrum confirmed the C-14/C15 double bond. A hydroxy and an acetate group were present at C-7α and C-12α respectively. The H-7 resonance at δ 4.0 was assigned based on HMBC correlation with C-5 and 3H-30. The H-12 resonance at δ 5.11 showed HMBC correlations with C-18 and C-17. The acetate methyl group proton singlet occurred at δ 1.89. 1H NMR COSY techniques verified coupling of H-17 and H-15 with H-16, H-9 and H-12 with H-11, H-22 with H-21 and H-23 and H-6 with H-5 and H-7. Stereochemistry was assigned on basis of the NOE experiments. The H-7 had a NOE correlation with 3H- 30 confirming that H-7 was β and thus, the hydroxy group was α. The H-12 had a NOE correlation with 3H-30 confirming that H-12 was β and thus the acetate group was in the α orientation. The 3Hβ-29 showed NOEs to H-6β, H-7β, H-12 β and H-15β. Further assignments were determined on the basis of HMQC and HMBC correlations. Compound 1 was characterized as 12α-acetoxy-1,2-dihydro-7-deacetylazadiron. It has previously been isolated from Turraea robusta [16].
Compound 2: Compound 2 was found to be α, β-unsaturated ketone of 1. This was indicated by a pair of doublets at δ 7.03 (J=10.2 Hz, H-1) and δ 5.83 (J=10.2 Hz, H-2) and resonances at δ 157.3, δ 125.7 and δ 204.8 ascribed to C1, C2, and C-3 respectively. It has been isolated as a synthetic derivative by reacting compound 1 with benzene seleninic anhydride and refluxing for thirty minutes in chlorobenzene [16]. This is the first report of its isolation from a natural source [17].
Compound 3: Compound 3 had an elemental formula of C30H42O7 (HREIMS m/z 514.1). Calculated value was found to be 514.6574. Peaks at m/z 454 [M-CH3COOH]+, 394 [M-2 (CH3COOH)]+ and 376 [M-2 (CH3COOH)-H2O]+ indicated the presence of two acetate groups and a hydroxyl in the molecule. Infrared (KBr) spectroscopy indicated the presence of hydroxyl (3558 cm-1), ester (1740 cm-1), ketone (1728 cm-1) and C-O (1245 cm-1). A Δ14-double bond was indicated by resonance at δ 158.6 and δ 122.6 for C-4 and C-15 respectively, and resonances ascribed to H-15 at δ 5.68. The limonoid β-substituted furan ring proton occurred at δ 7.33, 7.20 and 6.24 in the 500 1H NMR spectrum. Two hydroxyl groups were present at C-3β (δ 77.2) and C-7α (δ 71.8) respectively. The H-3 resonance at δ 2.26 was assigned based on HMBC correlation with C-1. The H-7 resonance at δ 3.96 was assigned based on HMBC correlation with C-5.
Two acetate groups were present C-1α (δ 74.7) and C-12α (δ 76.9) respectively. The H-1 resonance at δ 4.7 was assigned based on HMBC correlation with C-10 (δ 35.4) and C-5 (δ 40.4). The H-12 resonance at δ 5.08 was assigned based on HMBC correlation with C-17 (δ 50.1) and C-18 (δ 21.4). The relative stereochemistry at C-1, C-5, C-9 and C-12 was assigned based on the NOE experiments. The H-9α resonance was seen to correlate with 3H-18 and 3H-28 (which are α) and H-5 also showed a NOE correlation with the 3H-28. The H-1 and H-12 showed a NOE correlation with 3Hβ -19 confirming the α orientation of both the acetate groups at C-1 and C-12 respectively. The H-7 had a NOE correlation with 3H-30 confirming that H-7 was β and thus, the hydroxy group was α. The H-3 had a NOE correlation with 3H- 18 confirming that H-3 was α and thus, the hydroxy group was β. The H-17 resonance showed a NOE correlation with H-12β confirming the β-orientation of H-17. 1H NMR COSY techniques verified coupling of H-17 and H-15 with H-16, H-9 and H-12 with H-11, H-22 with H-21 and H-23, H-6 with H-5 and H-7 and H-2 with H-1 and H-3. Compound 3 is was characterized as 1α-12α-diacetoxy-1,2-dihydro-7- deacetyl-3β-7α-dihydroxyazadiron [17].
Two Meliaceae species were studied; Turraea abyssinica and T. cornucopia. This is the first time that T. cornucopia has been studied phytochemically. Three limonoids were isolated from T. cornucopia and classified as azadiron derivative type. The methanol and chloroform extracts exhibited potent larvicidal and adulticidal activities against A. gambiae. Delayed mortality was observed at low doses for all the methanol and chloroform extracts. These plants offer a significant potential for mosquito control as larvicides and adulticides. Due to the stability of the constituent limonoids against UV and oxygen, the extracts could be potential bed net impregnants.
This research was funded by WHO/MIM/TDR programme at ICIPE. We are grateful to Mr S.G. Mathenge for assistance with plant collection and identification. We are also grateful to Dr Antony of Rothamsted Research Hooper for running the NMR and MS spectra. Rothamsted Research receives grant-aided support from the Bio-technology and Biological Sciences Research Council (BBSRC).