Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 4
Plant species of the Sideritis genus are native to the Mediterranean region and widely used through millennia for their medicinal and culinary properties. They are mostly known as mountain tea or Shepard tea and in the last few years, they are on the spotlight due to the production of an enormous variety of bioactive secondary metabolites exerting a wide range of pharmaceutical applications. In the present study the biochemical value and the health promoting components of various indigenous Sideritis species was assessed: S. perforiata subsp. perfoliata, S. perfoliata subsp. athoa, S. syriaca, S. raeseri, S. scardica. A thorough biochemical analysis was conducted, including total phenols and flavonoid content, antioxidant activity, amino acids, elemental and polar/non polar metabolite profiling. S. perfoliata subsp. perfoliata had the most diverse and rich terpene profile among the studied species, while its infusion had the highest phenol and flavonoid content thus, showed the highest antioxidant activity. The diverse chemotype of Sideritis species can be used as a guide on the preparation of tea mixes with the desired metabolite content targeting specialized medical applications or may assist on the design of breeding strategies.
Sideritis; Mountain tea; Chemotype; Bioactivity
During the last few years, plants of the Labiatae family have been on the spotlight due to their unique properties. The genus Sideritis belongs to the Labiatae family and includes more than 150 species [1]. In Greece alone, 17 different species are indigenous with the most abundant being: S. perfoliata sub athoa, S. clandestina, S. scardica, S. raeseri, S. syriaca and S. euboea. Sideritis plants grow wild mainly in the mountains and only a few species are cultivated. Among them S. raeseri, S. scardica, S. syriaca and S. clandestina are found wild, as well as cultivated [2].
Sideritis species are rich in secondary metabolites with unique bioactive properties that are classified to terpenes, sterols, coumarins, flavonoids, iridoids and lignans [1,3]. Extensive research is carried out on the identification of the metabolites and exploration of their pharmacological properties. So far, Sideritis metabolites have been shown to exhibit antioxidant [4], anti-inflammatory [5], gastroprotective [6], neuroprotective [7,8], antimicrobial [9], analgesic [10], cardioprotective [11], anti-cancer, anti-HIV and anti-spasmolytic activity [1,12].
Several studies have investigated the pharmacological activity of Sideritis constituents administered as extracts or as food supplements.In an initial study by Kassi et al. [13], the potential preventive role of S. euboea and S. clandestina on osteoporosis was highlighted; the positive influence of S. euboea extracts on osteoblast was probably appointed to the flavonoid and phenylpropanoid glycosides [14]. Regular consumption of mountain tea has been proven to affect in a positive way the oxidant/antioxidant status of mice brain regions in a regio-selective and dose-dependent manner [15,16]. The synergy of S. scardica extract with vitamins of the B complex was shown to have a positive influence on alleviating the effects of chronic stress [17]. When S. euboea extract was administered orally in a jelly containing short-chain fructo-oligosaccharides a bifidogenic effect was observed [18]. Hofrichter et al. [7], found that extracts of S. scardica and S. euboea improved memory and learning in Alzheimer’s mouse models, while S. scardica extracts were proven to act as triple monoamine reuptake inhibitors [19] or exhibit an action as psychostimulant or antidepressive drug [20].
Despite the medicinal applications of Sideritis spp. and the consumers interest, limited studies have been conducted on the evaluation of the drinking tea [21] and correlation of its phytochemical composition with its bioactivity [14–16]. Since tea is amongst the most popular beverages, having custom made blends with specialized medicinal properties will benefit certain vulnerable consumers groups. The scope of the present paper was to assess the nutritional value of various indigenous Sideritis species through their infusions and identify superior chemotypes for drug discovery purposes and advanced plant breeding strategies.
Plant materials
Sideritis plants were collected from wild populations asexually propagated and cultivated in the same field. Details regarding species and sampling locations of the wild populations are presented in Table 1. The upper blossomed inflorescence of the plants (flowers, stems and leaves) was collected in the period of full flowering. After collection, the samples were air-dried away from direct sunlight and stored in paper bags at room temperature, on dry and dark place.
Chemicals
Standards of caffeic acid, p-coumaric acid, vanillic acid, transcinnamic acid, quercetin and ferulic acid were purchased from Fluorochem (Fluorochem Ltd, U.K.). 1,1-Diphenyl-2-picrlthydrazyl (DPPH) and Folin–Denis reagent were purchased from Sigma– Aldrich (Steinheim, Germany). BSTFA (N,O-bis(trimethylsilyl) trifluoroacetamide) along with solvents (HPLC or analytical grade) were purchased from PanReac Applichem (Barcelona, Spain). Trimethylchlorosilane (TMCS) was purchased from Aesar (Heysham, UK).
Tea infusion
Air dried plant materials were ground to a fine powder with a grinder. Tea infusions were prepared according to Kara et al. [22]. Briefly, 1 gr of dry material was soaked in boiled water for 5 min. Solid material was separated with filtration and the infusion was used for the following analyses: total phenol content, total flavonoid content, antioxidant activity and quantification of phenolic acids.
DPPH assay
The antioxidant activity of the infusions was estimated with the DPPH radical assay according to the method of Brand-Williams [23] as described by Boskou et al. [24] with minor modifications. An aliquot of the infusion (25 μL) containing different concentrations of the initial infusion was mixed with 975 μL of DPPH solution (6 × 10-5 M in methanol). The results are expressed as EC50 which is mg dry tissue/g of infusion required scavenging 50% of the initial concentration of the DPPH.
Total phenol content
Total Phenol Content of the infusions (TPC) was determined with Folin-Denis reagent according to the method described by Lanza et al. [25] with minor modifications. Briefly, 50 μL of tea infusion was mixed with 50 μL of Folin-Denis reagent. In the mixture 300 μL of a Na2CO3 saturated solution was added of and brought to 1 mL volume with d.H2O. The solution was allowed to stand for 60 min at room temperature. The mixture was centrifuged for 10 min at 3000 g and the absorbance of the supernatant was measured at 725 nm. The quantification of total phenols was determined by a calibration curve of caffeic acid ranging from 1-5 ppm with a regression coefficient value (R) of 0.9917. Total phenols were expressed as mg equivalents of caffeic acid /g of dry weight tissue (d.w.).
Total flavonoid content
Total Flavonoid Content of the infusions (TFC) was determined with AlCl3 colorimetric method as described by Zhinshen et al. [26]. The quantification of total flavonoids was determined by a calibration curve of quercetin ranging from 10-500 ppm with a regression coefficient value (R) of 0.9961. Total flavonoids were expressed as mg equivalents of quercetin /g of dry weight tissue (d.w.).
Elemental analysis
Nine trace elements (P, K, Ca, Mg, Fe, Mn, Zn, Cu, B) were measured with an inductively coupled plasma spectrophotometry, ICP-OES system (Thermo Scientific, USA). Briefly, the samples were incinerated at 460°C for 6 hours and the residue ashes were digested with 2N HCl, diluted up to 50 mL and measured with ICP-OES.
GC-MS analysis
Non-polar metabolites: Terpenes and terpenoids were analyzed according to a protocol of Tsaballa et al. [27]. All extracts were analyzed using a GC-2010 Plus Shimadzu gas chromatograph equipped with a GCMS-QP2010 Ultra gas chromatograph mass spectrometer, and a DB-5MS capillary column (30 m × 0.25 μm × 0.25 μm), in the splitless mode. The temperature of injector and detector was 250°C and 300°C, respectively. The oven temperature was slowly increased with a rate of 3°C/min from 60°C up to 240°C and maintained at this temperature for 5 min to equilibrate. Then the temperature was raised with 10°C/min at 290°C and kept isothermally for 10 min in order to elute compounds with higher boiling points. The carrier gas used for the analysis was helium at a flow rate of 1.3 mL/min. Mass spectra were acquired in a scan mode, while qualitative analysis was based on library search by using the following mass spectral libraries: FFNSC GC/MS Ver. 1.3 and Metabolite Component Database by Shimadzu, Wiley 7, NIST 11 and NIST 11 s.
Polar metabolites: For the chromatographic analysis of polar metabolites with GC- MS, a silylation of the polar metabolites of the tea infusions was performed employing BSTFA and TMCS reagents. Different parameters were examined in order to find the most suitable silylation conditions: extraction conditions, concentration and/or ratio of BSTFA/TMCS, reaction conditions. In order to determine the polar profile of the infusions, the 20 mL of the aqueous extract were liquid-liquid extracted with 3 × 10 mL of ethyl acetate. The organic layers were combined, dried over anhydrous Na2SO4 and evaporated to dryness under a stream of N2. BSTFA (200 μL) and 2 μL of TMCS were added and the reaction proceeded at room temperature for 1 hour. The silylated samples were analyzed using a GC-2010 Plus Shimadzu gas chromatograph equipped with a GCMS-QP2010 Ultra gas chromatograph mass spectrometer, and a DB-5MS capillary column (30 m × 0.25 μm × 0.25 μm), in the splitless mode. The GC program was according to Proestos et al. [28] with minor modifications. The temperature of injector and detector was set at 280°C and 220°C, respectively. The oven temperature was slowly increased with a rate of 4°C/min from 50°C up to 135°C and maintained at this temperature for 10 min. Then the temperature was raised with 4°C/min at 220°C and kept isothermally for 10 min. Finally, the temperature was raised at 300°C with a rate of 3.5°C/min and maintained at this temperature for 20 min in order to elute compounds with higher boiling points. The carrier gas used for the analysis was helium at a flow rate of 1.3 mL/min. Mass spectra were acquired in a scan mode, while qualitative analysis was based on library search by using the following mass spectral libraries: Metabolite Component Database by Shimadzu, Wiley 7, NIST 11 and NIST 11s.
Quantification of phenolic acids
Quantification of ferulic acid, caffeic acid, trans-cinnamic acid, vanillic acid and p- coumaric acid in the tea infusions was performed with a Flexar HPLC-DAD apparatus (Perkin Elmer, USA), using a Brownlee Bio C18 reverse-phase column (5 μm, 250 × 4.6 mm i.d.) (Perkin Elmer). The measurement of phenolic acids was performed according to the method of International Olive Council [29] with minor modifications. Briefly, the detection was performed at 260 nm, 280 nm, 310 and 325 nm. The elution solvents were A (0.2% H3PO4 in water), B (methanol) and C (acetonitrile). The samples were eluted according to the following gradient: 96% A, 2% B and 2% C for 1 min; 50% A, 25% B, 25% C in 30 min; 40% A, 30% B, 30% C in 5 min; 50% B, 50% C in 10 min and hold for 10 min; 96% A, 2% B and 2% C in 2 min and hold for 10 min. The flow rate was 1 ml/min and the run time 67 min. The identification of the compound was achieved by comparing their retention times with those of standards. The quantification of each compound was determined by a calibration curve: vanillic acid ranging from 25 to 400 ppm with a regression coefficient value (R) of 0.9959, caffeic acid ranging from 0.1 to 0.7 ppm with R=0.9949, p-coumaric acid ranging from 0.1 to 2 ppm with R value of 0.9918, ferulic acid ranging from 0.05 to 0.7 ppm with R value of 0.9978 and transcinnamic acid ranging from 5 to 100 ppm with R value of 0.9957.
Determination of amino acids
Amino acid content of the infusions was determined with EZFaastTM Free (Physiological) Amino Acid Analysis kit (Phenomenex) according to manufacturer’s instructions. All analysis was carried out in a GC-2010 Plus Shimadzu gas chromatograph equipped with a GCMS-QP2010 Ultra gas chromatograph mass spectrometer, and a ZB-AAA capillary column (10 m × 0.25 mm), in the split mode (split 1:15). The temperature of injector and detector was 240°C and 320°C, respectively. The oven temperature was slowly increased with a rate of 30°C/min from 110°C up to 320°C. The carrier gas used for the analysis was helium at a flow rate of 1.1 mL/min. Mass spectra was acquired in a scan mode, while qualitative analysis was based on library search by using the EZFaast library. The results are expressed as nmol of amino acid/ mL of infusion.
Principal component analysis
Evaluation of relatedness among Sideritis species was conducted by Principal Component Analysis (PCA) using a) all quantitative biochemical variables (TPC, TFC, DPPH, phenolic acids, amino acids, elemental) and b) only elemental analysis, since elemental content can be used as a taxonomic marker [30]. PCA was carried out in R environment [31] using ampvis2 [32] and ggplot2 R packages [33].
TPC, TFC and anti-oxidant activity of infusions Specimens of Sideritis species from different areas of Greece were collected and infusions from their aerial parts were prepared. Total phenol, total flavonoid content and antioxidant activity was measured in the prepared tea (Figure 1 and Supplementary Table 1).
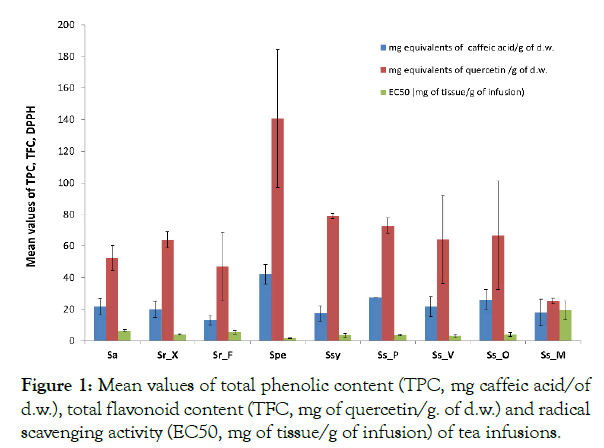
Figure 1: Mean values of total phenolic content (TPC, mg caffeic acid/of d.w.), total flavonoid content (TFC, mg of quercetin/g. of d.w.) and radical scavenging activity (EC50, mg of tissue/g of infusion) of tea infusions.
| Species | Location | Abbreviation |
|---|---|---|
| S. perfoliata | Trikala | Spe |
| S. perfoliata subsp. athoa | Pilima/Xanthi | Sa |
| S. scardica | Mt. Vermio | Ss_V |
| S. scardica | Mt. Olympos (1600m) | Ss_O |
| S. scardica | Mt. Menoikio | Ss_M |
| S. scardica | Moshopotamos/Pieria (~460m) | Ss_P |
| S. raeseri | Florina/Macedonia | Sr_F |
| S. raeseri | Kotyli/Xanthi | Sr_X |
| S. syriaca | Chania/Crete | Ssy |
Table 1: Sideritis species with their abbreviations.
S. perfoliata infusion (Spe) had the highest values of TPC (42.15 mg caffeic acid/g of d.w.) and TFC (140.71 mg quercetin/g of d.w.), while among S. scardica species TPC and TFC values ranged from 17.93 to 27.42 mg caffeic acid/g of d.w. and 25.14 to 72.86 mg quercetin/g of d.w., respectively. Among S. scardica spp, the high content of total phenols and flavonoids of Ss_O, Ss_P and Ss_V infusions was also depicted on their high antioxidant activity (Figure 1). S. raeseri from Florina had the lowest TPC value (13.11 mg caffeic acid/g of d.w.) while Ssy, Sr_X and Sa had similar values ranging from 17.26 (Ssy) to the higher 21.55 mg caffeic acid/g of d.w. of Sa. The highest antioxidant activity was displayed by Spe infusion and the lowest by the Ss_M infusion. All other species ranged from 3.22 to 19.33 mg of tissue/g of infusion
Phenol content of infusions
The derivatization of the infusions with BSTFA allowed the determination of terpene alcohols and acids along with phenolic acids, so on the heatmap both compound classes are presented (Figure 2). 2-phenylethanol and 4-OH benzoic acid, vanillic acid, ferulic, p-coumaric and caffeic acid were present in all the infusions. Cinnamic acid was identified in all samples but Ssy. Trans-cinnamic acid was hardly detected in the tea infusions, while ferulic acid and vanillic showed small fluctuations among species. Some analytes were identified only in specific species; the terpenic alcohols ascaridole and elemol were identified only in S. perfoliata infusion, 4-hydroxy cryptone and methyl dehydroabietate in Ssy infusion, dehydro-abietal in Ss_V and homovanillyl alcohol in Ss_O. The diterpenes labd-(13E)-8,15-diol was identified only in S. athoa. Amyrins, which are natural chemical compounds of the triterpene class, were identified in almost all samples but Sr_F, Ss_M and Spe.
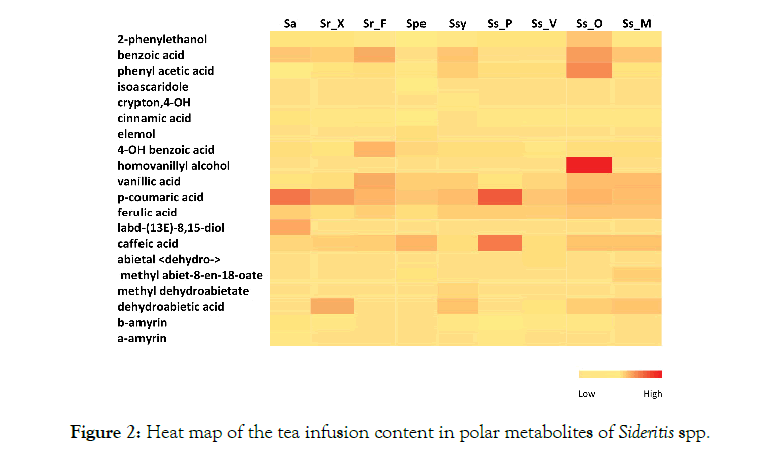
Figure 2: Heat map of the tea infusion content in polar metabolites of Sideritis spp.
The quantification of specific phenolic acids with HPLC-DAD provided a more accurate insight of the infusion content. As it is demonstrated on Table 2, Spe infusion had the highest amount of vanillic acid (28.565 mg/g of d.w.), followed by Ss_O and Ss_V infusions (23.368 and 18.45 mg/g of d.w., respectively). Caffeic acid concentration was similar among species with small fluctuations. However, p- coumaric acid varied among species, having high values in Sr_X and Ss_O (411.9 and 288.8 ppm μg/g of d.w., respectively) but 10 fold lower in other species ranging from 6.1 to 44.2 μg/g of d.w. Ferulic acid’s concentration was constant in the majority of infusions, with Sr_F having the highest concentration (30.7 μg/g of d.w.) and Spe infusion the lowest one (0.9 μg/g of d.w). Trans-cinnamic acid concentration showed a high variation among Sideritis species; Ss_P and Ss_V had similar values, while Ss_O had the highest concentration (15.926 g/g of d.w.) and Ss_M the lowest one (527.1 μg/g of d.w.).
| Vanillic acid | Caffeic acid | p-coumaric acid | Ferulic acid | Trans-cinnamic acid | |
|---|---|---|---|---|---|
| Sa | 4972.2 ± 252.3 | 14.3 ± 0.3 | 31.4 ± 8.1 | 16.9 ± 5 | 459.1 ± 101.6 |
| Sr_X | 3285.5 ± 911.3 | 9.8 ± 3 | 411.9 ± 39.6 | 13.4 ± 0.9 | N.Q. |
| Sr_F | 7393.7 ± 1356.7 | 8.7 ± 1.6 | 15.8 ± 0.1 | 30.7 ± 0.9 | N.Q. |
| Spe | 28565.1 ± 2988.1 | 7.5 ± 1.4 | 7.9 ± 2.0 | 0.9 ± 0.2 | N.Q. |
| Ssy | 3997.9 ± 725.7 | 10.9 ± 3.9 | 41.9 ± 13.5 | N.D. | N.D. |
| Ss_P | 3986.7 ± 2098.8 | 6.9 ± 1.5 | 44.2 ± 17.1 | 21.7 ± 4.6 | 2681.9 ± 118.3 |
| Ss_V | 18450.9 ± 96.50 | 14.21 ± 5.8 | 6.1 ± 0.4 | N.Q. | 2518.4 ± 338.7 |
| Ss_O | 23368.4 ± 1641.5 | 14.9 ± 2.5 | 288.8 ± 36.5 | 18.2 ± 3.1 | 15926.3 ± 1643.2 |
| Ss_M | 11870.9 ± 3563.4 | 9.4 ± 1.2 | 35.2 ± 6.1 | 16.9 ± 5.6 | 527.1 ± 175.3 |
Table 2: Concentration of main phenolic acids in tea infusions. Results are expressed as μg of phenolic acid/ g of dry weight ± S.D. (N.Q.: Not Quantified, N.D.: Not detected). Spe: S. perfoliata; Sa: S. perfoliata subsp. athoa; Ss_V: S. scardica; Ss_O: S. scardica; Ss_M: S. scardica; Ss_P: S. scardica; Sr_F: S. raeseri; Sr_X: S. raeseri; Ssy: S. syriaca.
Amino acid content of infusions
As it is shown on Figure 3, glycine and the aromatic amino acids phenylalanine, tyrosine and tryptophan had constant values among species with minor variation. Among branched-chain amino acids (BCAAs), leucine and isoleucine concentrations were constant among species with average values of 27.38 nmol/g to 12.74 nmol/g of infusion respectively, yet valine’s concentration showed high variability ranging from 34.25 nmol/g of infusion (Ss_V) to 109.26 nmol/g of infusion (Sr_X). It’s noteworthy, that in Ssy infusion, tyrosine and tryptophan were not detected. On the other hand, threonine values are highly fluctuated among species, ranging from 1.05 nmol/g of infusion at Sa to 17.22 and 18.89 nmol/g of infusion at Sr_F and Ss_M, respectively. Yet, it was not detected at Ssy and Ss_P. In a similar manner, serine values varied between 42.18 at Sa to approximately 110 nmol/g of infusion at Sr_F and Ss_O. Sa and Ss_O contain the highest concentration of proline; 130.38 and 159.55 nmol/g of infusion, respectively. The lowest value was recorded at Ssy_Ch (31.44 nmol/g of infusion). High variability was also observed in asparagine values, ranging from a low 167.16 nmol/g of infusion at Spe to a high 914.71 nmol/g of infusion at Sr_X. Ss_V had the highest concentration of aspartic and glutamic acid (28.82 and 124.32 nmol/g of infusion, respectively) while Spe had the lowest value of aspartic acid (6.32 nmol/g of infusion) and Sr_X the lowest value of glutamic acid (36.53 nmol/g of infusion). The highest concentration of alanine was measured at the infusions of Sr_F, Spe and Ss_O (133.83, 112.87 and 110.99 nmol/g of infusion, respectively) while the other tissues had an average value of 71.44 nmol/g of infusion. The non-protein b-aminoisobutyric acid (B-AIb), had an average value of 13.17 nmol/g of infusion, with small fluctuations among species. The highest values were recorded for Ss_O (15.09 nmol/g of infusion) as well as for Sr_F and Sa (14.73 and 14.60 nmol/g of infusion, respectively).
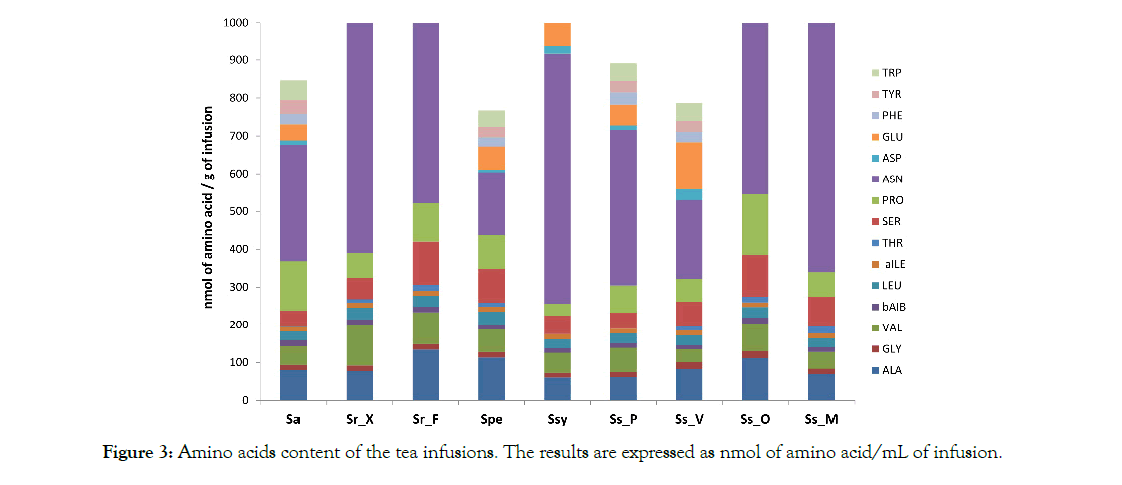
Figure 3: Amino acids content of the tea infusions. The results are expressed as nmol of amino acid/mL of infusion.
Terpenes profile
Terpene and terpenoid profile of the hexane extracts of Sideritis species was obtained with gas-chromatography-mass spectrometry. As it is shown on Table 3, Sideritis species are rich in terpenes, with S. perfoliata subsp. perfoliata having the highest heterogeneity; more than 90 terpenes and terpenoids were identified. Among monoterpenes, the most common compounds were a-terpineol, trans- pinocarveol, trans-verbenol, pinocarvone and verbenone. S. perfoliata’s extract was rich in monoterpenes compared to the other species; a- and b-pinene, b-bisabolene, a-elemol, b-eudesmol, valeranone, b-chamigrene, 8-a-acetoelemol and occidenol were the main metabolites. Besides Spe, other tissues also contain unique monoterpenes, for instance S. syriaca (b-phellandere, cuminal), and Ss_M (Mentha- 2,8-dien-1-ol <trans-, p-). The most common sesquiterpenes among species were b- elemene, b-bourbonene, trans-caryophyllene and its oxide, b-bergamotene, germacrene d-cubebol, calamine, trans-cadin-1,4-diene and a-bisabolol. It’s noteworthy, that a-elemol’s concentration in the Spe extract reaches approximately 9%. Some S. scardica species are characterized by unique sesquiterpenes, for instance b-cubebene and 10-epicubebol are only identified in Ss_O, trans-muurola- 4(14),5-diene in Ss_V, and d-cadinenene and cis-muurol-5-en-4-alpha-ol only in Ss_P while trans-pinocarveol, trans-verbenol and myrtenol are mainly identified in Ss_M. Rich in unique sesquiterpenes was also the extract of S. syriaca having b-cadinene, b-sesquiphellandrene, a-bisabolene and copaborneol. Regarding diterpenes, the most common compounds among species were geranyl-a-terpinene, pimaradiene, geranyl-p-cymene, kaure-15-ene, 3-a-hydroxy-manool, abienol and thunbergol.
| Sa | Sr_X | Sr_F | Spe | Ssy | Ss_P | Ss_V | Ss_O | Ss_M | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (Area %) | ||||||||||
| R.t. (min) | Compound | |||||||||
| Monoterpenes | ||||||||||
| 5.35 | a-Pinene | 1.86 | ||||||||
| 6.52 | b-Pinene | 0.47 | ||||||||
| 6.37 | Sabinene | 0.03 | 0.11 | |||||||
| 7.40 | 3-Carene | 0.02 | ||||||||
| 7.93 | p-Cymene | 0.08 | ||||||||
| 8.06 | dl-Limonene | 0.13 | ||||||||
| 8.13 | b-Phellandrene | 1.12 | ||||||||
| 9.05 | g-Terpinene | 0.07 | ||||||||
| 9.22 | Trans Sabinene hydrate | 0.02 | 0.19 | 0.1 | ||||||
| 10.30 | Linalool | 0.06 | ||||||||
| 10.60 | Camphene hydrate | 0.11 | ||||||||
| 10.93 | Mentha-2,8-dien-1-ol <trans-, p-> | 0.1 | ||||||||
| 10.94 | Thujol | 0.01 | ||||||||
| 11.88 | trans-Pinocarveol | 0.05 | 0.03 | 0.12 | ||||||
| 12.09 | trans-Verbenol | 0.06 | 0.06 | 0.17 | ||||||
| 12.74 | Pinocarvone | 0.03 | 0.01 | 0.04 | ||||||
| 13.14 | Borneol | 0.06 | 0.01 | |||||||
| 13.77 | p-Cymen-alpha-ol | 0.06 | ||||||||
| 14.08 | Cryptone | 0.64 | 0.12 | |||||||
| 14.11 | a-Terpineol | 0.04 | 0.17 | 0.08 | 0.35 | 0.45 | ||||
| 14.45 | (-)-Myrtenol | 0.06 | 0.31 | |||||||
| 14.60 | Verbenone | 0.02 | 0.03 | 0.1 | ||||||
| 15.12 | cis-Carveol | 0.04 | 0.02 | |||||||
| 16.42 | Cuminal | 0.19 | ||||||||
| 17.09 | Ascaridole | 0.03 | ||||||||
| 17.73 | p-Mentha-1,5-dien-7-ol | 0.04 | ||||||||
| 18.55 | p-Cymen-7-ol | 0.02 | 0.24 | |||||||
| 18.82 | Piperitone | 0.04 | ||||||||
Table 3: Terpene profile of Sideritis species.
Elemental analysis
Elemental analysis of the aerial parts (Table 4), showed that the concentration of phosphorous ranged from 0.16% (Spe) to 0.41% (Ss_O) with an average value across samples of 0.26%. The concentration of potassium and magnesium showed small variations with an average value of 1.6% and 0.29%, respectively. High variation was observed on the values of calcium, ranging from 0.57% (Sr_X) to 3.8% (Ssy_Ch). The average concentration of plants in sodium was 741 mg/Kg, with Ss_P having the highest value. The variation of iron content was very high, with Ss_P having a 5-fold higher concentration from the lowest of Sa (98.2 mg/Kg). Similar variation was observed to borium content, ranging from 8.1 mg/Kg at Sa to 42.1 mg/Kg at Spe. Small variations were observed on the values of manganese, zinc and copper. It’s noteworthy, that S. perfoliata subsp. athoa had the lowest values of the majority of minerals while S. scardica species had the highest values in most of the studied elements.
| P% | K% | Ca% | Mg% | Na (mg/Kg) | Fe (mg/Kg) | Mn (mg/Kg) | Zn (mg/Kg) | Cu (mg/Kg) | B (mg/Kg) | |
| Sa | 0.19 | 1.2 | 0.92 | 0.15 | 660 | 98.2 | 21.3 | 20.5 | 14.2 | 8.1 |
| Sr_X | 0.23 | 1.6 | 0.57 | 0.21 | 704 | 108 | 42.2 | 28.5 | 13 | 15.8 |
| Sr_F | 0.24 | 1.5 | 0.86 | 0.34 | 672 | 145 | 32.3 | 25.4 | 29.5 | 21.5 |
| Spe | 0.16 | 1.3 | 1.3 | 0.29 | 763 | 115 | 28.6 | 25.5 | 17.5 | 42.1 |
| Ssy | 0.26 | 1.1 | 3.8 | 0.33 | 699 | 193 | 46.1 | 35.3 | 21.3 | 32.7 |
| Ss_P | 0.27 | 2.1 | 0.72 | 0.24 | 1001 | 470 | 32.8 | 26.8 | 18.4 | 18.5 |
| Ss_V | 0.33 | 1.5 | 1.5 | 0.45 | 768 | 262 | 52.7 | 42.5 | 21.7 | 41.3 |
| Ss_O | 0.41 | 2.5 | 0.72 | 0.22 | 764 | 184 | 46 | 28.4 | 21.8 | 28.8 |
| Ss_M | 0.3 | 1.8 | 0.97 | 0.34 | 641 | 136 | 43.4 | 20.9 | 16.4 | 34.3 |
Table 4: Elemental analysis of Sideritis species. Spe: S. perfoliata; Sa: S. perfoliata subsp. athoa; Ss_V: S. scardica; Ss_O: S. scardica; Ss_M: S. scardica; Ss_P: S. scardica; Sr_F: S. raeseri; Sr_X: S. raeseri; Ssy: S. syriaca.
Principal component analysis
In order to evaluate the biochemical profile and calculate distances between Sideritis species based on the variables obtained/analyzed, principal component analysis was applied. The terpene profile of each plant was not included in the correlation study because only absolute quantifications were used. PCA on all biochemical variables did not reveal any distinct grouping of species (Figure 4A). The first two principal components accounted for a high percentage of the total variation in both cases (89.7% and 94.3% for all biochemical variables and only for elemental analysis, respectively).
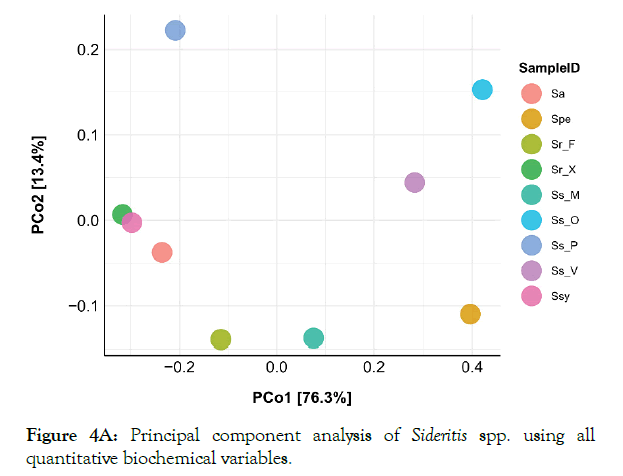
Figure 4A: Principal component analysis of Sideritis spp. using all quantitative biochemical variables.
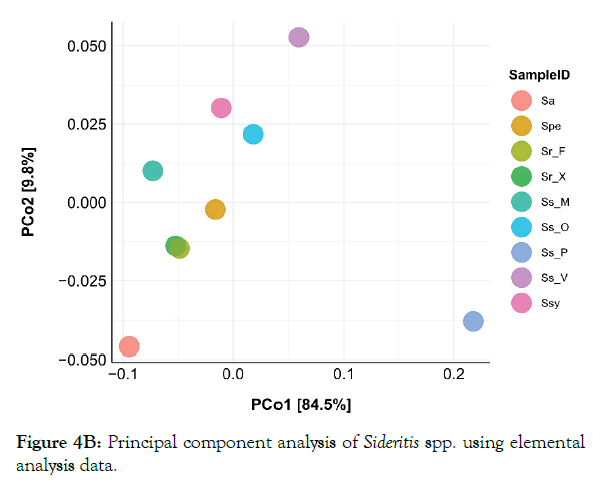
Figure 4B: Principal component analysis of Sideritis spp. using elemental analysis data.
When all biochemical markers were analyzed a loose clustering of species was observed (Figure 4A). Even among wild populations of the same species (e.g. S. scardica) PCA did not group samples according to their origin. In addition, Ss_P was found to be more differentiated from all the other samples, whereas Sr_X and Ssy appear to have a closer relation compared to other species. However, this was not the case when grouping of species was assessed solely on elemental analysis. The former analysis revealed that Ss_P is the most differentiated species, located away from all the other samples (Figure 4B). Finally, elemental analysis grouped very tightly Ss_X and Sr_F species.
Tea is amongst the most popular beverages consumed worldwide and has been shown to have health promoting properties. So far, evaluation of the health promoting properties of mountain tea has been limited to specific species such as S. scardica, S. raeseri, S. clandestine and S. euboea [7,13–16,18–20]. In general, Sideritis medicinal properties are being mainly evaluated as extracts or supplements with emphasis on metabolite classes [14,16] than specific compounds [15].
Due to the popularity of tea, knowing the biochemical profile of various Sideritis spp. can either guide us on the preparation of tea mixes with the desired metabolite content targeting specific applications e.g. for memory enhancement, or can assist on superior chemotype breeding strategies. To address this, the biochemical profile of indigenous Sideritis spp. was thoroughly investigated. Among the tested infusions, S. perfoliata’s subsp. perfoliata had the highest phenol and flavonoid content thus, showing the highest antioxidant activity which is in agreement with previous findings [5]. The TPC values of S. scardica infusions were similar to previous reports [21] and lower than others [34], while regarding TFC values S. scardica from Mt. Menoikio was similar to previous reports [21] but from Pieria, Mt. Vermio and Mt. Olympus had higher values. In the case of S. raeseri values, TPC was lower compared to previous findings [34–36] but comparable to others [37,38]. In addition, the TFC value of the infusion was higher than previously reported [35,38]. Regarding antioxidant activity, S. raeseri and S. scardica infusions exhibited analogous activity to a previously reported one [34]. In the case of S. syriaca infusion, total phenol content was in agreement to Goulas et al. [39].
S. scardica samples were low in monoterpenes that is similar to reports from the Balkans [40,41] but had significant amounts of sesquiterpenes such as trans- caryophyllene, germacrene, bicylogermacrene, trans-caryophyllene, cadinene derivatives as well as sesquiterpenic alcohols as spathulenol and cadine-4-en- 10-ol. Among S. scardica samples, Mt. Menoikio had significant amounts of trans- pinocarveol, trans-verbenol and myrtenol that were shown to act as potent modifiers of GABA receptor function [42]. S. raeseri samples were also poor in monoterpenes but were rich in the sesquiterpenes trans-caryophyllene and its oxide, bicyclogermacrene and a-bisabolol; that is in agreement with previous studies [3]. Previous reports on S. raeseri and S. scardica identified bicyclogermacrene and spathulenol as the main constituents of the latter [34,36]. Among diterpenes, S. raeseri extracts were rich in kaurene derivatives and that is in agreement with previous studies [34,36]. S. perfoliata subsp. perfoliata had the most diverse and rich terpene profile among the studied species, with a-elemol being the most abundant compound (Table 3). The high concentration of elemol could be attributed to a heat induced rearrangement of hedycaryol [43], however this hypothesis is not supported since its presence was also detected as a TMS derivative formed under mild conditions (Figure 2).
Besides terpenes and terpenoids, phenolics represent the next class of Sideritis metabolites having medicinal properties. Derivatization with BSTFA and TMCS, allowed the identification of the phenol profile of the infusions as well as the discovery of hydroxylated terpenes. The relevant abundance of phenolics showed a fluctuation among species (Figure 2). However, some TMS-derivatives of the phenolic acids where not detected with HPLC; this difference can be attributed to the different sensitivity of each method as well as to the different pre-treatment of the sample. Among S. scardica samples, caffeic and ferulic acid were comparable to previous reports while vanillic acid was much higher [21]. Regarding transcinnamic acid, Ss_P and Ss_V had comparable values to Irakli et al. while p-coumaric values were much lower [21].
To our knowledge this is the first time that the amino acid content of infusions has been quantified. The chromatographic analysis highlighted the amino acid heterogeneity among species pointing to their diverseness. Highest variability was observed to the values of valine, threonine, serine, proline, asparagine, aspartic and glutamic acid. The beneficial effect of Sideritis tea on the Central Nervous System (CNS) and on the alleviation of symptoms related to neurodegenerative disease may be attributed to the amino acid content since the role of specific amino acids has been associated. For instance, the role of glutamic and aspartic acid in the CNS has been proved [44] while valine has been associated with diseases such as diabetes, influence the immune and the central nervous system [45]. The relieving effect of mountain tea on gastrointestinal disorders might be attributed to the different content of asparagine, since it was shown to alleviate intestinal inflammation [46]. Or even the different action of Sideritis infusion on obesity and metabolic disorder may be attributed to the different content of b-aminoisobutyric acid [47].
The role of amino acids on plant metabolism [48] and response to various stresses [49] have been well documented and in the present study is depicted on the heterogeneity of the infusion content. However, it is not clear whether this heterogeneity is stress induced or/and is species related. Nevertheless, this information is valuable as allows the regulation of the health promoting constituents of tea infusions in a stress-oriented manner. Not all species of Sideritis have the same effect on the central nervous system and cognitive functioning and this is mainly attributed to the different chemotype. Consequently, it’s important to assess the chemotype of the drinking infusion as to exert the maximum beneficial affect from its tea.
Since tea next to water is the most popular non-alcoholic beverage, the assessment of it mineral content is essential for nutritional value assessment. Elemental analysis was performed on the aerial parts, as the extractability of elements depends on the nature of the element and on the infusion conditions such as temperature and steeping time [30]. Among macrominerals (P, K, Ca, Mg), potassium was the most abundant among species as expected [21,36] and calcium [3]. Sodium was the most abundant in all plants while in the case of micro-minerals, in all species the order was Fe>Mn>Zn. In S. scardica, the order was Fe>Mn>Zn>B>Cu which is different from previous reports [21]. Sodium content of Sideritis spp. was similar to previous reports while zinc values were double [50]. Compared to Karapandzova et al. [34], the values of sodium were higher while ferrous content was alike. In another study, copper and ferrous values were much higher while boron content of S. raeseri from Xanthi was alike [50]. Among micro-elements, ferrous had the highest concentration and this is in agreement with previous observations in S. raeseri [36]. These differences between different studies could be attributed to the different content of minerals in the dry tissue and in the infusions. Furthermore, the soil composition and pH, the agricultural practices, the geographical origin and the variety have been proved to affect the mineral content of the plants [30].
The principal component analysis of the datasets revealed a dispersion of the different members of the Sideritis genus depicting differences both in genetic level and on geographical origin of the initial wild populations. Furthermore, S. raeseri from Xanthi and S. syriaca are grouped together (Figure 4A), implying a similar chemotype. From the PCA plot (elemental), the grouping of some S. scardica and S. raeseri samples implied a differentiation within genus. This grouping is not surprising since S. scardica and S. syriaca are phylogenetically close [51,52].
The biochemical fingerprint of Sideritis species highlighted both their nutritional value and chemical diversity providing valuable information for potent applications. The metabolite profile of mountain tea was shown to be both species and geographical origin dependent. Since mountain tea is a popular beverage, having available the chemotype of different species will assist in a dual way; on a targeted selection of tea blends for specialized medical applications or to their utilization as supplements but also on the design of breeding strategies.
The authors declare that they have no competing interests.
F. A. Trikka is a recipient of a post-doctoral fellowship from the Greek State Scholarships Foundation (ΙΚΥ) in the context of the project “Reinforcement of Postdoctoral Researchers” (MIS- 5001552) that is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Program «Human Resources Development, Education and Lifelong Learning». This research was funded from the Trans Adriatic Pipeline in the context of the “Thriving Land” project. The authors would like to thank Dr. E. Maloupa for kindly providing Sideritis samples.
Citation: Trikka F, Michailidou S, Makris AM, Argiriou A (2019) Biochemical Fingerprint of Greek Sideritis spp.: implications for Potential Drug Discovery and Advanced Breeding Strategies. Med Aromat Plants (Los Angeles) 8.335. doi: 10.35248/2167-0412.19.8.335
Received: 19-Jul-2019 Accepted: 29-Jul-2019 Published: 05-Aug-2019 , DOI: 10.35248/2167-0412.19.8.335
Copyright: © 2019 Trikka F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.