
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2021)
Joint infections with non-specific presentation are still difficult to diagnose. We sought to identify biochemical markers in Snovial Fluid (SF) that can predict susceptibility to ongoing inflammatory processes in the joint cavity. Ninety-two consecutive patients were divided into four SF groups based on clustering analysis: non-inflammatory SF (73%), inflammatory-non-pyogenic (12%), inflammatory-pyogenic (10%), or hemorrhagic (5%). We measured and compared the levels of the following biochemical markers in SF: glucose, lactate, total protein, uric acid, C-Reactive Protein (CRP), Leukocyte Count (WBC), Mononuclear (MNP), Polymorphonuclear (PMN), Interleukin (IL)-1 beta, IL6, Procalcitonin, Presepsin, Neutrophil Gelatinase-Associated Lipocalin (NGAL), Human Neutrophil Defensin 1-3 (HNP1-3), Cartilage Oligomeric Matrix Protein, Lactoferrin (HLF2), Polymorphonuclear Elastase (PMNE), Matrix Metalloproteinase (MMP)-1, and MMP-3. Discriminant analysis predicted the classification of individual SF samples into the relevant SF groups with an accuracy of 94.4%. We found a significant difference between WBC, PMN, MNP, CRP, IL-1β, IL-6, HNP1-3, HLF2, PMNE, and individual groups of SF type (p0.6; p0.6; p<0.0001), and between PMN and MNP in the inflammatory-non-pyogenic and inflammatory-pyogenic SF groups (rs= -1.000; p<0.0001). PMN, MNP, WBC, CRP, and HNP1-3 in SF predicted the inflammatory processes with excellent diagnostic performance. The combination of these SF biomarkers can contribute to earlier diagnosis of the inflammatory process in the joint cavity.
Synovial fluid, Protein, Inflammatory Biomarkers, Immunoassay
Joint infections occurring with an overall change in the patient's condition and a typical joint puncture are usually not a diagnostic problem. However, joint infections with a non-specific presentation are difficult to diagnose, mainly due to the absence of specific clinical signs and symptoms, relative lack of accurate laboratory tests, low virulence due to previous treatment, and biofilm ability of the pathogens. This is especially true for patients treated with non-targeted antibiotics, including patients with implanted joint replacements. Therefore, new biochemical markers are being sought to help quickly determine the extent of the inflammatory process taking place in the joint cavity in routine biochemical practice, either due to its increased concentration in synovial fluid (SF) or directly in serum/plasma.
Processes that occur inside the joint have been shown to be a combination of inflammatory catabolic and co-occurring anti-inflammatory anabolic processes that are mediated at the cytokine level [1]. In the present study, we looked at two inflammatory cytokines that control ongoing disease, interleukin (IL)-1 beta and IL-6. IL-1β is synthesized by chondrocytes, osteoblasts, synovial membrane-forming cells, and mononuclear cells during the inflammatory response, independently evoking inflammatory responses and catabolic effects [2-7]. IL-1β also affects the activity of chondrocytes in the synthesis of enzymes, such as metalloproteinases (MMPs). The groups of MMPs are mainly interstitial collagenase (MMP-1), stromelysin-1 (MMP-3), and collagenase 3 (MMP-13), which has a destructive effect on components of cartilage [8-10]. Chondrocytes exposed to IL-1β also tend to age more rapidly and induce apoptosis [11-13]. IL-1β also inhibits cartilage regeneration. In joint cells, IL-1β is able to induce its own secretion in an autocrine manner to stimulate the synthesis of other cytokines, such as TNF-α, IL-6, IL-8, and CCL5 chemokines [14-18].
IL-6 strongly activates the immune system and increases the inflammatory response, which can be evaluated as an anti-inflammatory interaction due to some of its effects. The synthesis of IL-6 in the joint is usually a response to IL-1β and TNF-α, involving chondrocytes, osteoblasts, fibroblast synoviocytes, macrophages, and adipocytes [1]. IL-6 is present at elevated concentrations in both SF and serum [19-21]. The effect of IL-6 on the articular cartilage is a decrease in the production of type II collagen and an increase in the production of MMP enzymes [22]. These effects have also been found to be potentiated by injury [23]. IL-6 is considered a key cytokine that causes changes in the subchondral bone layer. Its effect is largely based on the promotion of osteoclast formation and, thus, bone resorption, but exhibits synergism with IL-1β and TNF-α [24-26]. Osteoblasts stimulated by IL-1β, TNF, and IL-6 become their source and may also produce MMPs by adversely affecting cartilage in their vicinity [26].
Another of the studied biomarkers is Human Neutrophil Defensin (HNP1-3). These peptides are localized on azurophilic granules. They have microbicidal effects, simultaneously exhibiting chemotactic, immunomodulatory, and cytotoxic activity, and are involved in host defense and inflammation. Activation of neutrophils leads to the rapid release of defensins into the SF and serum/plasma. Under physiological conditions, plasma HNP1-3 concentrations are very low, often undetectable. Under septic conditions, however, the concentrations increase significantly, reaching values of upto 10 g.L-1 [27-30].
Cartilage oligomeric matrix protein (COMP) is a non-collagenous glycoprotein with a molecular weight of 524 kDa. COMP belongs to the thrombospondin family of extracellular proteins and binds Ca2+. COMP is present in the extracellular matrix of articular, nasal, and tracheal cartilage, and in tendons, meniscus, ligaments, and synovium [31-33]. COMP has been shown to be released into the SF during erosive joint diseases, and elevated levels can also be observed in serum [31]. COMP is a valuable tool for identifying patients at high risk of rapid joint destruction and for monitoring the effectiveness of treatments [34,35]. In addition, recent studies have suggested that COMP is a potential biomarker of arthritic diseases, such as systemic sclerosis [36,37], liver disease [38,39], and breast cancer [40].
Polymorphonuclear Elastase (PMNE) localizes in azurophilic granules of polymorphonuclear granulocytes. During the phagocytosis of foreign substances, PMNE is excreted into the extracellular environment, where its activity is regulated by inhibitors, especially the α1-proteinase inhibitor (α1-PI). Enzymatically active PMNE, together with co-produced oxidants (O2 radicals, H2O2, OH radicals), can cause local tissue damage. α1-PI forms a complex with all secreted elastases. The concentration of the PMNE/α1-PI complex correlates with the released PMNE and can be used as a measure of granulocyte activity during the inflammatory response. The PMNE test is used to monitor the course of trauma, shock, and sepsis, as well as joint diseases and exudates in sports injuries [41].
Neutrophil gelatinase-associated lipocalin (NGAL) is a member of the lipocalin protein family and has a molecular weight of 21 kDa. NGAL has been discovered in specific neutrophil granules, where it is covalently bound to gelatinase. Its role in the natural defense against bacterial infection has been attributed to its ability to interfere with the absorption of bacterial iron by competing with the siderophore of enterobactin. Thus, NGAL can be considered a potential marker of bacterial infection. This biomarker also a described ability to distinguish between bacterial and viral infection [42-44].
Lactoferrin is an iron-binding glycoprotein that belongs to the transferrin family. Its iron-binding capacity is twice as large as that of transferrin. Lactoferrin was isolated from breast milk and subsequently identified in exocrine gland secretions and specific neutrophil granules [45,46]. HLF2 plays a role in iron metabolism, cell proliferation, and differentiation, and exhibits antibacterial, antiviral, and antiparasitic activity and catalytic, antitumor, anti-inflammatory, anti-allergic, and radioprotective functions and properties. It is considered a marker of neutrophil granulocyte activation and considered by some to be an acute phase protein [47-49].
The aim of the present study was to verify the division of patient groups by SF and measure the concentration of the selected biomarkers in the different groups to find the most suitable biomarker or groups of biomarkers for discriminating inflammatory and non-inflammatory joint infection.
Patients
Our study included 92 patients from the Orthopedic Department whose synovial fluid and serum samples were sent for analysis at the Institute of Laboratory Diagnostics, Department of Clinical Biochemistry, University Hospital Ostrava. Clinical criteria for inclusion: age >18 years, sufficient amount of synovial fluid obtained during a joint or burst puncture; clinical criteria for exclusion: age less than 18 years, insufficient synovial fluid (less than 5 ml), suspected aspiration of venous or arterial blood, patients treated with an oncology program for systemic chemotherapy, patients at risk of post-puncture bleeding (full anticoagulant therapy at the time of puncture, or congenital or acquired hypocoagulation conditions with insufficient substitution), pregnancy in women. All patients signed informed consent prior to their involvement in the study. Approval was obtained from the Ethics Committee of the University Hospital Ostrava, Czech Republic (reference number of project 322/2018) in accordance with the Helsinki Declaration of 1975 as revised in 2000. Patient samples were divided into individual groups of SF according to Dungl et al. [50]: non-inflammatory SF (35 women, average age 67.2 ± 14.5 years; 32 men, average age 55.4 ± 15.3 years), inflammatory - non-pyogenic (due to the emphasis on the absence of a pyogenic form of inflammation; 5 women, average age 49.6 ± 18.5 years; 6 men, average age 58.2 ± 18.8 years), inflammatory-pyogenic (2 women, average age 71.5 ± 7.8 years; 7 men, average age 59.3 ± 12.3 years) and hemorrhagic SF (1 women, age 70 year; 4 men, average age 73.0 ± 14.7 years).
The duration of the disease, comorbidity (diabetes mellitus, high blood pressure, cancer, depressive disorder, etc.). And associated medication were monitored, however, no effects on the tests we had used were found, which is consistent with the general use of these types of tests. The 1st quartile of the duration of the disease was 8.5 days, the median was 41.5 days and the 3rd quartiles were 102.75 days. The range was 1-1840 days.
Samples
SF samples were collected in a polypropylene tube (Sarstedt, Nümbrecht, Germany). A portion of the non-centrifuged SF sample was used for cytology. The remainder of the SF sample was centrifuged at 2500 g for 6 minutes at 4°C and the supernatants aliquoted into three to five vials (0.5 ml per vial) and stored at -70°C until analysis.
Analytical methods
The concentrations of the following biomarkers were determined on an AU 5800 automated analyzer using references from Beckman Coulter (Brea, CA, USA): glucose (REF OSR6521), lactate (REF OSR6193), total protein (REF OSR6132), uric acid (REF OSR6298), and C-reactive protein (CRP; REF OSR6147). We also analyzed hematological parameters, relative and absolute numbers of leukocytes (WBC), and mononuclear (MNP) and polymorphonuclear (PMN) leukocyte counts on a Sysmex XN-9000 Automated in Body Fluid mode. IL-6 (ADVIA Centaur IL6, REF 10995080, Siemens) and procalcitonin (PCT; ADVIA Centaur BRAHMS Procalcitonin, REF 10378883, Siemens) were determined on an Advia Centaur XP automated analyzer and presepsin (PRES; Pathfast Presepsin, REF 1110-4000, Mitsubishi Kagaku Iatron, Inc.) on a Pathfast system. We used enzyme-linked immunosorbent assay (ELISA) to measure IL-1β (Human Interleukin-1 Beta ELISA, REF RD194559200R, BioVendor Research and Diagnostic Products), HNP1-3 (Human HNP1-3 ELISA KIT, REF HK317, HycultBiotech, Inc., United States), lactoferrin (HLF2; Human Lactoferrin ELISA, REF. RD194334200R, BioVendor Research and Diagnostic Products), NGAL (Human Lipocalin-2/NGAL ELISA, REF RD191102200R, BioVendor Research and Diagnostic Products), COMP (Human Cartilage Oligomeric Matrix Protein ELISA, REF RD194080200, BioVendor Research and Diagnostic Products), PMNE (Human PMN Elastase ELISA, REF RM191021100, BioVendor Research and Diagnostic Products), MMP-1 (Human MMP1 ELISA Kit, REF ab100603, ABCAM), and MMP-3 (Human Matrix Metalloproteinase-3 ELISA, REF RD191510100CS, BioVendor Research and Diagnostic Products).
Undiluted ST were used to determine routine and inflammatory biomarkers on the automated analyzer using 1/10 diluted samples for PRES, 1/3 diluted samples for IL-1β, 1/1000 diluted samples for HNP1-3, 1/500 diluted samples for HLF2, 1/500 diluted samples for PMNE, 1/100 diluted samples for NGAL, 1/25 diluted samples for COMP, 1/25 diluted samples for MMP-3, and 1/20 diluted samples for MMP-1.
Statistical analysis
Statistical analyses were performed using Stata version 13, MedCal version 17.9.7., R, and QC. Expert 3.3.6.6 – Trilobyte Statistical Software [51]. Basic descriptive statistics, including medians and percentiles, were used to describe the results.
The division of patients into individual diagnostic groups was verified by cluster and discriminant analysis. The original groups were difficult to separate in multidimensional space. For better separation into individual diagnostic groups, we used the K means algorithm for clustering. The optimal number of clusters was determined by the Elbow method.
We performed principal component analysis (PCA) to reduce the original variables to a smaller number of latent variables (principal components). Multinomial logistic regression and discriminant analysis were performed to determine the accuracy of the prediction classification.
The normality of the SF parameters was verified by the Shapiro-Wilk test of normality. The normality hypothesis was rejected; therefore, non-parametric tests were used, including the Kruskal-Wallis rank test (i.e., ANOVA). The relationship between the parameters was evaluated by Spearman's Correlation Coefficient (rs), with rs > 0.6 considered a strong positive or negative relationship.
The diagnostics value of SF biomarkers was evaluated by receiver operating characteristics (ROC) curve analysis. When calculating the sensitivity and specificity (and 95% confidence interval [CI]) of each SF biomarker, the results of the non-inflammatory group were considered against the group involving any inflammatory process. A biomarker with an area under the curve (AUC) > 0.9 was considered to have excellent diagnostic power, and a biomarker with an AUC ≤ 0.5 represented a biomarker without diagnostic power. The significance level for statistical tests was set at p = 0.05.
The characteristics of the 92 patients included in the analysis are presented in Table 1.
| Median | Mean | SD | Min | Max | |
|---|---|---|---|---|---|
| Age (years) | 62 | 61.3 | 15.9 | 18 | 94 |
| WBC (×109 L-1) | 0.48 | 6.36 | 13.6 | 0.01 | 68.4 |
| PMN (%) | 32.2 | 41.2 | 28.1 | 2.4 | 97 |
| MNP (%) | 0.24 | 1.31 | 3.06 | 0.009 | 23.9 |
| PMNA (×109 L-1) | 0.09 | 5.04 | 12.4 | 0.01 | 65.5 |
| MNP (×109 L-1) | 67.9 | 58.8 | 28.1 | 3 | 97.6 |
| pH (-) | 8 | 7.7 | 0.5 | 6 | 9 |
| UA (μmol.L-1) | 297 | 324 | 113 | 142 | 719 |
| LAC (mmol.L-1) | 2.98 | 3.74 | 2.63 | 0.01 | 14.8 |
| CRP (mg.L-1) | 1.85 | 10.8 | 23 | 0.01 | 137 |
| IL-1β (ng.L-1) | 1.89 | 16.1 | 47.9 | 0.4 | 301 |
| IL-6 (µg.L-1) | 1.01 | 9.56 | 18.6 | 0.003 | 55 |
| PCT (µg.L-1) | 0.66 | 0.67 | 0.19 | 0.01 | 1.4 |
| PRES (µg.L-1) | 2.29 | 3.78 | 4.17 | 0.05 | 21.9 |
| HNP1-3 (µg.L-1) | 219 | 1823 | 3371 | 0.156 | 19,300 |
| NGAL (µg.L-1) | 45.6 | 189 | 448 | 0.02 | 3650 |
| HLF2 (µg.L-1) | 41.6 | 3754 | 9751 | 1.1 | 73,542 |
| COMP (µg.L-1) | 2330 | 2557 | 1173 | 186 | 9428 |
| MMP-1 (µg.L-1) | 52.5 | 184 | 294 | 1.12 | 1410 |
| MMP-3 (µg.L-1) | 218 | 213 | 71.2 | 19 | 347 |
| PMNE (mg.L-1) | 5.94 | 255 | 546 | 0.72 | 2550 |
WBC, leukocytes; PMN, polymorphonuclear; MNP, mononuclear; UA, uric acid; LAC, lactate; CRP, C-reactive protein; IL, interleukin; PCT, procalcitonin; PRES, presepsin; HNP1-3, human neutrophil defensin 1-3; HLF2, lactoferrin; NGAL, neutrophil gelatinase-associated lipocalin; MMP, matrix metalloproteinase; COMP, cartilage oligomeric matrix protein; PMNE, polymorphonuclear elastase
Table 1: Descriptive characteristics of the studied patients; N=92.
The division of patient samples into individual diagnostic groups was verified by cluster analysis. According to the Elbow method, the optimal number of clusters for our data was 4: cluster 1, non-inflammatory SF samples (73%); cluster 2, hemorrhagic (5%); cluster 3, inflammatory-non-pyogenic (12%); and cluster 4, inflammatory-pyogenic (10%, Figure 1). An overview of the biochemical markers that distinguish the clusters from one another is given in Table 2.
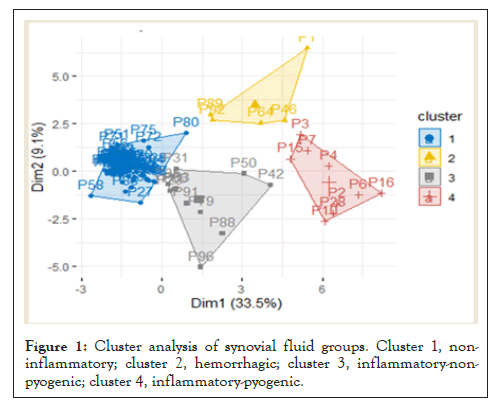
Figure 1: Cluster analysis of synovial fluid groups. Cluster 1, noninflammatory; cluster 2, hemorrhagic; cluster 3, inflammatory-nonpyogenic; cluster 4, inflammatory-pyogenic.
| Cluster | |||
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| (N = 67) | (N = 5) | (N = 11) | (N = 9) |
| MNP | IL-1b | MMP-1 | PMNA |
| pH | HNP1-3 | MNA | WBC |
| COMP | HLF2 | MMP-3 | IL-6 |
| MMP-3 | IL-6 | PMN | PMNE |
| PMNE | CRP | PMN | |
| PRES | WBC | HNP1-3 | |
| LAC | LAC | CRP | |
| PMN | PMNA | NGAL | |
| CRP | UA | LAC | |
| MMP-3 | pH | HLF2 | |
| NGAL | PMNA | PCT | |
| MNA | |||
| IL-1b | |||
Table 2: The level of inflammatory factors of this COVID-19 patient.
The basic characteristics of the individual groups after including the results of the cluster analysis are given in Table 3.
| Synovial fluid group | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age (years) | 62 | 70 | 53 | 64 |
| 15.6 to 89.3 | 59.0 to 92.0 | 29.0 to 94.0 | 39.0 to 78.0 | |
| WBC (×109 L-1) | 0.25 | 1.05 | 12 | 39.6 |
| 0.05 to 10.8 | 0.37 to 19.1 | 3.19 to 33.7 | 6.8 to 68.4 | |
| PMN (%) | 25.8 | 60 | 72 | 92.4 |
| 4.71 to 67.0 | 38.6 to 95.4 | 52.1 to 95.9 | 85.4 to 97.0 | |
| MNP (%) | 0.17 | 0.31 | 2.57 | 2.27 |
| 0.03 to 7.62 | 0.17 to 0.88 | 0.49 to 23.9 | 0.75 to 5.16 | |
| PMNA (×109 L-1) | 0.04 | 0.46 | 8.85 | 37 |
| 0.01 to 3.63 | 0.15 to 18.3 | 2.33 to 22.3 | 6.01 to 65.5 | |
| MNP (×109 L-1) | 74.2 | 40 | 28 | 7.6 |
| 33.0 to 95.3 | 4.60 to 61.4 | 4.1 to 47.9 | 3.00 to 14.6 | |
| pH (-) | 8 | 8 | 8 | 8 |
| 7.0 to 8.0 | 6.0 to 8.0 | 7.0 to 8.0 | 7.0 to 8.0 | |
| UA (μmol.L-1) | 298 | 316 | 277 | 286 |
| 147 to 675 | 160 to 435 | 179 to 556 | 177 to 566 | |
| LAC (mmol.L-1) | 2.78 | 6.59 | 3.62 | 6.9 |
| 0.10 to 6.43 | 3.38 to 10.0 | 2.10 to 14.8 | 3.20 to 11.1 | |
| CRP (mg.L-1) | 1.1 | 0.8 | 9.8 | 39 |
| 0.10 to 13.9 | 0.04 to 64.0 | 3.40 to 95.1 | 5.10 to 137 | |
| IL-1β (ng.L-1) | 1.25 | 224 | 1.9 | 36.1 |
| 0.40 to 7.41 | 107 to 301 | 1.25 to 25.6 | 12.1 to 71.2 | |
| IL-6 (µg.L-1) | 0.471 | 36.5 | 2.63 | 55 |
| 0.005 to 17.9 | 5.50 to 55.0 | 0.065 to 40.2 | 51.0 to 55.0 | |
| PCT (µg.L-1) | 0.7 | 0.7 | 0.56 | 0.6 |
| 0.50 to 0.99 | 0.01 to 0.90 | 0.16 to 0.70 | 0.50 to 1.40 | |
| PRES (µg.L-1) | 2.33 | 2.97 | 1.94 | 1.9 |
| 0.55 to 14.5 | 0.75 to 21.4 | 0.59 to 14.3 | 0.71 to 4.47 | |
| HNP1-3 (µg.L-1) | 96 | 8420 | 1130 | 7200 |
| 0.156 to 6789 | 2120 to 19,300 | 116 to 7340 | 5170 to 8910 | |
| NGAL (µg.L-1) | 34.1 | 372 | 86.8 | 777 |
| 0.02 to 411 | 10.1 to 894 | 10.3 to 698 | 387 to 1098 | |
| HLF2 (µg.L-1) | 2.5 | 9230 | 1316 | 20,659 |
| 1.10 to 4462 | 81.2 to 73,542 | 1.10 to 6347 | 80.8 to 32,993 | |
| COMP (µg.L-1) | 2409 | 2714 | 2484 | 1678 |
| 1497 to 7714 | 186 to 3027 | 1532 to 3498 | 1304 to 2746 | |
| MMP-1 (µg.L-1) | 48.3 | 50.3 | 256 | 18 |
| 4.20 to 567 | 32.0 to 557 | 43.0 to 1410 | 18.0 to 808 | |
| MMP-3 (µg.L-1) | 224 | 232 | 226 | 131 |
| 67.9 to 335 | 138 to 325 | 95.3 to 316 | 119 to 169 | |
| PMNE (mg.L-1) | 4.66 | 985 | 78.7 | 1590 |
| 0.85 to 572 | 1.66 to 1530 | 1.29 to 1310 | 303 to 2550 | |
Table 3: Descriptive characteristics of the individual synovial fluid groups.
Data are given as medians and 2.5th to 97.5th percentiles. Groups: 1, non-inflammatory; 2, hemorrhagic; 3, inflammatory-non-pyogenic; 4, inflammatory-pyogenic. WBC, leukocytes; PMN, polymorphonuclear; MNP, mononuclear; UA, uric acid; LAC, lactate; CRP, C-reactive protein; IL, interleukin; PCT, procalcitonin; PRES, presepsin; HNP1-3, human neutrophil defensin 1-3; HLF2, lactoferrin; NGAL, neutrophil gelatinase-associated lipocalin; MMP, matrix metalloproteinase; COMP, cartilage oligomeric matrix protein; PMNE, Polymorphonuclear elastase.
PCA replaced the original 20 biomarkers with 10 latent biomarkers which explain 87.2% of the total variation. This analysis allowed us to find the most suitable biomarkers for the differentiation of individual SF groups and their representation in clusters (Figure 2). The contributions of the variables to the first and second main components are shown in Figure 3. Further analysis included SF biomarkers with a contribution > 5%: WBC, IL-6, PMNA, PMNE, MNP, PMN, HLF2, IL-1β, HNP1-3, and CRP.
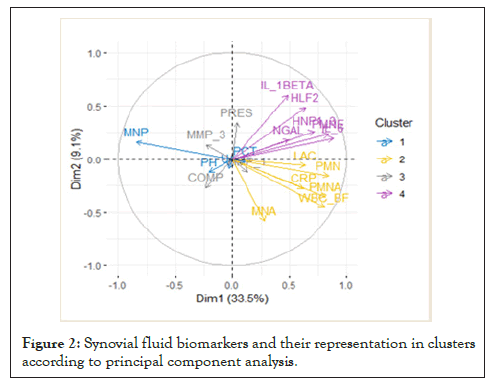
Figure 2: Synovial fluid biomarkers and their representation in clusters according to principal component analysis.
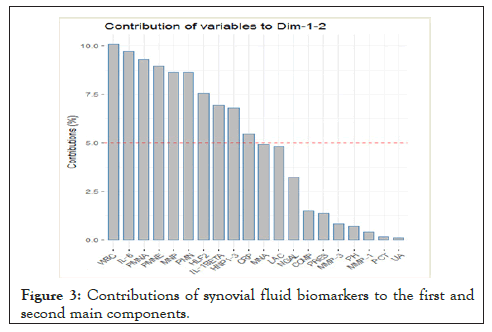
Figure 3: Contributions of synovial fluid biomarkers to the first and second main components.
The multinomial logistic regression showed that the accuracy of predicting the inclusion of individual SF samples into the relevant SF groups was 77%, but the discriminant analysis prediction was much better, 94.4%. For this reason, the discriminant analysis is more appropriate for classifying other patients.
A significant difference was found by the non-parametric Kruskal-Wallis test between WBC, PMN, MNP, CRP, IL-1β, IL-6, HNP1-3, HLF2, PMNE, and individual SF groups (p<0.0001, Figure 4).
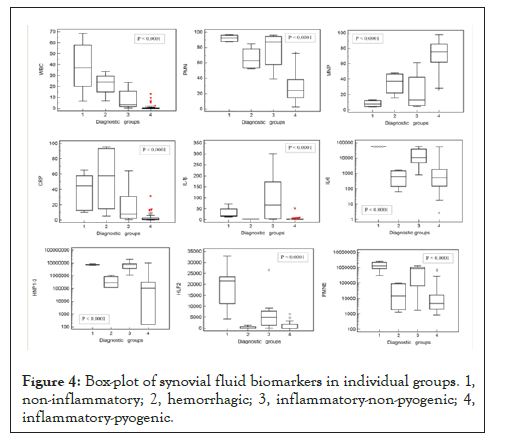
Figure 4: Box-plot of synovial fluid biomarkers in individual groups. 1, non-inflammatory; 2, hemorrhagic; 3, inflammatory-non-pyogenic; 4, inflammatory-pyogenic.
We found a significant positive correlation between WBC and PMN, MNP, and CRP concentrations; between PMN and HNP1-3 and PMNE concentrations; between IL-6 and PMNE concentrations; between IL-1β and NGAL, HLF2, and PMNE concentrations; between HNP1-3 and NGAL, HLF2, and PMNE concentrations; between NGAL and HLF2 and PMNE concentrations; and between HLF2 and PMNE concentrations (rs > 0.6; p<0.0001, Supplemental table 1).
We evaluated the correlation between the individual biomarker concentrations and the different SF groups. We also found a significant positive correlation between WBC and MNP concentrations; between IL-1β and NGAL and MMP-3 concentrations; between HNP1-3 and PMNE concentrations; and between NGAL and HLF2 concentrations in the non-inflammatory SF group (rs > 0.6; p<0.0001, Supplemental table 2), and a significant negative correlation between PMN and MNP in the inflammatory-non-pyogenic SF group and inflammatory-pyogenic SF group (rs = -1.000; p<0.0001, Supplemental table 3 and Supplemental table 4). The correlations between individual biomarkers in the hemorrhagic SF group were not assessed due to the small number of samples.
Five biomarkers in the SF (PMN, MNP, WBC, CRP, and HNP1-3) predicted the inflammatory processes with excellent diagnostic power (AUC > 0.9, Figure 5A). The sensitivity and specificity of these biomarkers were 90-100% (Table 4). Six additional biomarkers (PMNE, NGAL, IL-6, IL-1β, LAC, and HLF2) demonstrated AUC values between 0.8 and 0.9%. The diagnostic power of these biomarkers was lower, but when assessing their correlation with the strongest predictors of the inflammatory process (WBC, PNM, MNA, and HNP1-3) they showed a strong positive correlation.
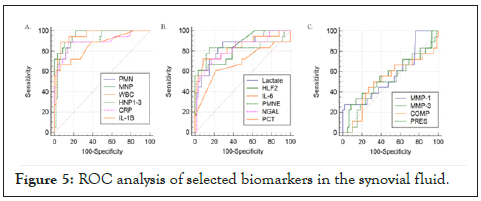
Figure 5: ROC analysis of selected biomarkers in the synovial fluid.
| AUC | P | Sensitivity (%) | Specificity (%) | Cut-off | |
|---|---|---|---|---|---|
| PMN | 0.989 | <0.001 | 100 | 92.5 | 48.90% |
| MNP | 0.989 | <0.001 | 100 | 92.5 | 47.90% |
| WBC | 0.986 | <0.001 | 100 | 94 | 2.20 ×109 L-1 |
| CRP | 0.952 | <0.001 | 100 | 82.1 | 3.0 mg.L-1 |
| HNP1-3 | 0.91 | <0.001 | 90 | 86.6 | 586 µg.L-1 |
| PMNE | 0.889 | <0.001 | 90 | 88.1 | 53.7 mg.L-1 |
| NGAL | 0.866 | <0.001 | 70 | 92.5 | 106.8 µg.L-1 |
| IL-6 | 0.861 | <0.001 | 75 | 91 | 2.5 µg.L-1 |
| IL-1β | 0.813 | <0.001 | 55 | 94 | 5.54 ng.L-1 |
| LAC | 0.809 | <0.001 | 65 | 86.6 | 4.51 mmol.L-1 |
| HLF2 | 0.807 | <0.001 | 55 | 97 | 3.2 µg.L-1 |
| PCT | 0.646 | 0.079 | 75 | 58.2 | 0.64 µg.L-1 |
| MMP-3 | 0.646 | 0.046 | 65 | 62.7 | 206 µg.L-1 |
| COMP | 0.619 | 0.131 | 40 | 88.1 | 1870 µg.L-1 |
| MMP-1 | 0.593 | 0.248 | 30 | 100 | 678 µg.L-1 |
| PRES | 0.537 | 0.606 | 55 | 59.7 | 1940 ng.L-1 |
| UA | 0.518 | 0.829 | 40 | 77.6 | 256 µmol.L-1 |
AUC, area under the curve; PMN, polymorphonuclear; MNP, mononuclear; IL, interleukin; WBC, leukocytes; HLF2, lactoferrin; CRP, C-reactive protein; NGAL, neutrophil gelatinase-associated lipocalin; HNP1-3, human neutrophil defensin 1-3; LAC, lactate; MMP, matrix metalloproteinase; COMP, cartilage oligomeric matrix protein; PCT, procalcitonin; PRES, presepsin; PMNE, polymorphonuclear elastase; UA, uric acid
Table 4: Sensitivity and specificity of synovial fluid biomarkers.
In this study, we evaluated 20 biomarkers in the SF as potential candidates for the diagnosis of infection. Using linear discriminant analysis, we obtained the coefficients of the discriminant function, which was able to classify a new patient or an SF sample into a relevant group with an accuracy of 94.4%. At the same time, PCA selected 10 biomarkers with more suitable properties, and these parameters correlated with one another.
Similar results were obtained by Deirmengian et al. [52], who studied SF biomarkers in patients after revision arthroplasty of the hip or knee joint, including patients with systemic inflammatory disease. They showed that cytokines and proteins with antimicrobial functions play an important role in the innate response to the elimination of pathogens, such as alpha-defensins (e.g., HNP1-3), neutrophil elastase 2, bactericidal/permeability-increasing protein, NGAL, and HLF2, providing the greatest utility for the diagnosis of periprosthetic join infection (PJI). Our study tested patients with various types of joint effusion, including non-inflammatory, predominantly in patients with OA, non-pyogenic inflammation, and pyogenic inflammation. All cases had increased concentrations of these biomarkers, which increased with the severity of the inflammatory process. Unfortunately, due to the diversity of the SF groups, we did not achieve the excellent results of the other studies.
Deirmeingian et al. were the first to demonstrate that NGAL exhibits 100% sensitivity and specificity when categorized according to the Musculoskeletal Infection Society (MSIS) criteria [52]. Vergara et al. demonstrated 86% sensitivity and 77% specificity of NGAL [53]. Their cut-off value for distinguishing aseptic failure versus established infection was 152 µg.L-1, with a median NGAL in patients with sepsis vs an aseptic course of approximately 1536 µg.L-1 vs 87 µg.L-1. In the present study, we demonstrated 70% sensitivity and 92.5% specificity of NGAL in distinguishing aseptic and septic infection. The median NGAL in patients with pyogenic inflammation was 777 µg.L-1 vs 87 µg.L-1 in inflammatory-non-pyogenic joint infections. These partial differences could be due to different methods of measurement. In the study by Vergar et al., a modified ELISA with chemiluminescence detection on an ARCHITECT i 1000SR analyzer from Abbott Laboratories was used to compare the ELISA assay from BioVendor Research and Diagnostic Products used in our study.
Another test with high diagnostic efficiency was WBC, especially the PMN count in SF, both with 100% sensitivity and a specificity of 92.5% and 94%, respectively. The cut-off value for distinguishing between inflammatory and non-inflammatory type SF was 2.20×109 L-1 and 48.9% cells, respectively. Due to their high diagnostic accuracy, these parameters were also exceeded by CRP, HNP1-3, or the cytokines IL-6 and IL-1β and proved to be more suitable biomarkers for differentiating the inflammatory process in the joint cavity. This is supported by the results of a meta-analysis [54] that reported a sensitivity of 89% and a specificity of 86% for WBC, with cut-off values ranging from 1100×106 L-1 to 27,800 ×106 L-1 according to the MSIS criteria for a group of patients with PJI. Thanks to the high diagnostic efficiency of these tests and rapid analysis, we can talk about first-choice laboratory biomarkers for distinguishing between inflammatory and non-inflammatory processes in the joint cavity.
Similarly, the acute inflammatory response marker, CRP, which had 100% sensitivity and 82% specificity with a cut off value of 3 mgL-1, could be included among the first-choice biomarkers. In addition, cytokines that stimulate the synthesis of acute phase proteins (IL-6, IL-1β, and others) had much lower sensitivity (55-75%) but higher specificity (91-94%).
We measured elevated levels of IL-1β in the inflammatory non-pyogenic (1.25 - 25.6 ng.L-1) and inflammatory pyogenic (12.1 - 71.2 ng.L-1) groups vs. the non-inflammatory group, which mainly comprised OA type (0.40 - 7.41 ng.L-1). A similar issue was addressed by Sohn et al. [55], who compared the levels of different cytokines in patients with rheumatoid arthritis (RA) and OA. They demonstrated higher levels of IL-1β in RA patients compared to OA in both SF and serum. Median IL-1β and IL-6 levels in the SF of patients with OA was comparable to our results (1.14 vs 1.25 ng.L-1 and 975.4 ng.L-1vs 471 ng.L-1, respectively).
Inflammatory cytokines with a primarily destructive effect on articular cartilage, such as IL-1β and IL-6, also contribute to the increased synthesis and release of many proteolytic enzymes, such as MMPs, which break down articular cartilage. Two of them, MMP-1 and MMP-3, were identified in this study. The low sensitivity of these biomarkers (30% and 60%) precluded their use in distinguishing between inflammatory and non-inflammatory joint effusion. However, Heard et al. [56] found elevated levels of MMP-1 and MMP-3 in samples from patients with advanced OA compared to samples with early OA, and significantly increased levels of MMP-1 in advanced OA compared to normal samples of SF. They also showed a significant increase in MMP-1 and MMP-3 levels in RA patients compared to normal samples. The differences may be due to different groups of patients tested, or a different method of measurement (luminescence detection vs. chromogenic detection).
Another of the studied biomarkers was COMP. Our data showed very low diagnostic efficiency, probably due to this biomarker being used to monitor the progress of cartilage degradation. Lorenco et al. [57] studied the clinical efficacy of total COMP and COMP neoepitope in a group of patients with RA, reactive arthritis, OA, or acute trauma. They found elevated concentrations in all groups. However, the ratio of the neoepitope COMP to total COMP was also significant. The authors showed significant differences between groups of patients and between patients with RA with slow or rapid progression of joint damage. This may be related to the different sequence of events in the catabolism of this molecule depending on the nature of the disease. As COMP is also present in tendons, ligaments, and synovium, these tissues may also contribute to increased concentrations of COMP in SF.
A very promising biomarker for differentiating the inflammatory nature of SF appears to be lactoferrin, which had good diagnostic strength with an AUC > 0.8.
There was a significant relationship between lactoferrin and both WBC and PMN, probably due to the fact that lactoferrin is released from activated neutrophils at the site of inflammation. At the same time, a strong correlation was demonstrated between lactoferrin and the cytokines IL-1β and IL-6, as well as other potential biomarkers of inflammation (HNP1-3, NGAL, and PMNE). Wong [58] studied this biomarker in patients with RA and found that lactoferrin levels in the SF do not correlate with disease severity, but with a marker of inflammation, specifically CRP. Similar conclusions were reached by Bennett [59], who determined the levels of lactoferrin and lysozyme in the SF of patients with traumatic effusions, OA, RA, pseudogout, septic arthritis, and gout, graded according to neutrophil count. They found elevated lysozyme levels in all patients with inflammatory arthritis and OA. However, lactoferrin concentrations were not elevated in OA, but correlated with the extent of the inflammatory response because, unlike lysozyme, which arises from both cartilage and neutrophils, lactoferrin is derived only from neutrophils.
Wang et al. [60] studied SF biomarkers in patients with PJI and found three promising proteins for the diagnosis of PJI, including lactoferrin. Using mass spectrometry, the AUC for lactoferrin was 0.9888. The results were also compared by ELISA and verified that HLF2 is not only sufficiently sensitive, but also specific for the diagnosis of PJI.
Another intensely studied SF parameter is the alpha-defensins, which are released very quickly into the SF after neutrophil activation. In this study, excellent diagnostic performance of this parameter was demonstrated for the diagnosis of infection. This parameter was ranked among the five most effective biomarkers, with 90% sensitivity and 86.6% specificity. Melicherčík et al. [61] also studied this marker in SF from 157 patients diagnosed with a joint infection, such as PJI, infectious arthritis, arthrosis, reactive arthritis, and RA. The concentration of HNP1-3 was determined by HPLC, which had 94% sensitivity and 92% specificity for the diagnosis of PJI and 97% sensitivity and 87% specificity for the diagnosis of infectious arthritis. The diagnosis of PJI using alpha-defensins was also discussed by Suda [62] in their study of 28 patients who underwent removal of total hip arthroplasty (THA) or total knee replacement (TKR). Alpha-defensin had comparable sensitivity (76.9%) and specificity (82.4%) with our study, though we did not study patients with PJI.
PMNE also appears to be a suitable biomarker for distinguishing the inflammatory character of joint effusion, with a sensitivity of 90%, specificity of 88.1%, and a cut-off value of 53.7 mgL-1. Momohara discussed the significance and mechanism of action of PMNE in patients with RA [63], showing increased PMNE activity in the damage joints. They concluded that PMNE could play a significant role in RA.
This study has some limitations. Although three to five aliquots of SF were usually frozen for each patient, there was not always enough material for all assays, especially when the analysis had to be repeated to find an analyte concentration outside the calibration range. In addition, some samples of SF were so viscous that they could not be used for all analyses, especially on fully automated systems due to the risk of clogs.
SF biomarkers WBC, PMN, MNP CRP, HNP1-3, IL-1b, IL-6, PMNE, and HLF2 allow the classification of new patients into the relevant SF group with an accuracy of 94.4%. In addition, WBC, PMN, MNP, CRP, and HNP1-3 provide excellent diagnostic sensitivity and specificity for the diagnosis of infection, despite the study including a limited number of patients with pyogenic and non-pyogenic inflammation. The data obtained in this study show the importance of monitoring joint diseases with more than one biomarker to enhance their diagnostic effectiveness. We think that the combination of these SF biomarkers can contribute to earlier diagnosis of the joint inflammatory process.
Designed the study: PK, IB, PW, and RH. Performed and evaluated biochemical markers: PK and IB. Clinical data collection and management: PK, IB, PW, and RH. Analyzed the data: PK, IB, PW, RH, and FV. Wrote the first draft of the manuscript: PK. Editing of the manuscript: IB, PW, RH, FV, and DS. Read and approved the final version of the manuscript: PK, IB, PW, RH, and DS.
This study was supported by the Ministry of Health, Czech Republic – conceptual development of research organization (FNOs/2018). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
We thank Ing. F. Všianský for help with the statistical analysis.
Citation: Bystro?ov�¡ I, Ku�¡nierov�¡ P, Walder P, Hlubek R, Stejskal D (2021) Biochemical Markers of Differentiation of the Inflammatory Process in Synovial Fluid. J Clin Chem Lab Med. 4:161.
Received: 22-Feb-2021 Accepted: 08-Mar-2021 Published: 15-Mar-2021
Copyright: �© 2021 Bystro?ov�¡ I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.