Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2016) Volume 5, Issue 2
Injection of heat killed Escherichia coli into the 5th instar of Schitocerca gregaria lead to deleterious effect on macromolecules such as proteins, lipids, and DNA, which expressed in the form of increased levels of carbonyls, peroxides, strand breaks, respectively. The results showed significant difference, in macromolecules damage, between control insect and injected one through time course and application of different concentration of E. coli. The present results were focusing on properties of lipopolysaccaride of bacterial cell wall which cause production of nitric oxide radical as an immune response of insect and also can react with superoxide anion radical that form peroxynitrite that considered as reactive nitrogen species which cause macromolecules damage Also, the results showed that the antioxidant enzymes activities, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) were elevated over constitutive levels, in response to the injected oxidative stressor heat killed E. coli. The levels of both the constitutive and induced activities of GPx and are always higher in whole midgut homogenate than in thoracic muscles. The results may represent some sort of stressful conditions that may increase the vulnerability of insect pests to control measures.
<Keywords: Antioxidant enzymes; Lipid peroxidation; Protein carbonylation; Alkaline comet assay; Schistocerca gregaria ; Lipopolysaccarides
However, under environmental stress, e.g. bacterial infections, ROS levels may increase dramatically, resulting in significant damage to cell structures. This process is known as oxidative stress [1,2]. The cells of native animals are able to defend themselves against ROS damage through the use of antioxidants. Various antioxidants may for example decrease the level of lipid peroxidation as well as DNA and protein damage [3,4]. Of the major components of the antioxidant defense system of insects include several antioxidant enzymes, such as superoxide dismutases (SOD), catalases (CAT), peroxidases (POX), glutathione-S-transferase (GST) [3]. Excess generation of reactive oxygen species (ROS) including free radicals, e.g. superoxide anion radical (O2-), hydroxyl radical (OH), and hydroperoxyl radical (HO2) may cause oxidative stress. Free radicals are highly reactive, unstable molecules [5], and their breakdown can produce non-radical reactive species, such hydrogen peroxide (H2O2) and peroxynitrite (ONOO-) [6-10]. It was found that reactive nitrogen species (RNS) can be produced during immune response of insect in the form of nitric oxide radical (NO) that interact with O2- and generate (ONOO-) [11].
In this case, oxidative stress may result when an imbalance takes place between ROS production and antioxidants ability to restore the normal homeostatic condition [6,8,10,12]. Consequently, injection of heat killed Escherichia coli can result in indirect damages to cell macromolecules by the produced ROS [13,14]. From these oxidative damages of ROS are those of the cell lipids, proteins, and DNA in the form of lipid peroxides, protein carbonyls, and DNA strand breaks respectively.
The resulting oxidative damage of the cell macromolecules can be reduced by a system of antioxidant defense including enzymatic and non-enzymatic mechanisms [3,15-18]. This system scavenges ROS, repairs damages, and degrades the non-repairable ones. Antioxidant enzymes are important preventive antioxidants which act to reduce the formation of ROS and increase cell survival [19,12]. The principal enzymes involved are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR).
The present work aimed to evaluate the oxidation effect of the injected heat killed bacteria E.coli , and the antioxidant response in the thoracic muscles and whole midgut of 5th instar S.gregaria . Macromolecules such as proteins, lipids, and DNA were measured in the form of protein carbonyls, lipid peroxides, and DNA strand breaks, respectively as a result of injected heat killed E. coli. Also, the constitutive and induced activities of four principal antioxidant enzymes, SOD, CAT, GPx, and GR, as an enzymatic antioxidant response system were assessed.
The insect and tissues
Desert locusts, S.gregaria (Forskal), were from a well-established laboratory colony at the Entomology Department, Faculty of Science, Cairo University, Egypt. The insects were reared in wooden cages (60 cm×60 cm×40 cm) at 30 ± 2°C, 60 ± 5 RH, and 16:8 h (Light: Dark) photoperiod. Locusts were fed on fresh alfalfa, Medicago sativa (Papillioacea) and 5th instar individuals were used for experiments.
Oxidative damage assays
Protein carbonyls were assayed according to the method of the lipid peroxides concentration was measured according to [20], and the alkaline comet assay was used to assess the DNA strand breaks and carried out according to [21].
Antioxidant enzyme assays
Approximately, 2.5 gm tissue samples were homogenized in 2.5 ml ice-cold phosphate buffer. The homogenates were centrifuged at 10,000 g for 30 min at 4°C, and an aliquot from each of supernatant was used for assessing enzyme activities.
The SOD activity was measured following [22], and expressed as OD/μg protein/min. For the activity of CAT was determined according to [23], and expressed as OD/μg protein/min.
The activity of GPx was determined according to [24], and expressed as OD/μg protein/min. The activity of GR was determined according to [25], and expressed as OD/μg protein/min. The total protein concentration of samples was determined spectrophotometrically according to the method of [26], using bovine serum albumin (BSA) as standard.
Statistical analysis
The significant differences of the treated groups with respect to control were analyzed by independent-t-test, and one-way analysis of variance (ANOVA). The Tukey's post hoc Test was applied and significant levels were set at p<0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows (Version 17.0. Armonk, NY: IBM Corp.). The difference among the thoracic muscles and whole midgut were also analyzed using independent-t-test. Data were expressed as mean ± SE.
Oxidative damage
Protein carbonyls: Different concentrations of heat killed E. coli that caused the highest production of protein carbonyls (as an indicator of oxidative stress) were tested and the optimum ones were selected; 12 cell / 15 μl saline/ individual for this stressor (Table 1).
| Concentration of the E.coli (cell/15µl) | |||||||
|---|---|---|---|---|---|---|---|
| Tissue | 0 | 6 | 12 | 24 | 30 | 36 | |
| Protein carbonyls | Muscle | 0.477±0.013Aa | 0.150±0.006Ab | 0.263±0.018Ab | 2.290±0.063Ac | 0.186±0.002Ab | 0.193±0.001Ab |
| Midgut | 0.768±0.015Ba | 0.177±0.014Bc | 0.235±0.118Bc | 2.749±0.013Bd | 0.256±0.003Bc | 0.268±0.006Bc | |
| Lipid peroxides | Muscle | 0.004±0.002Aa | 12.490±0.864Ab | 16.032±0.600Ab | 68.731±2.770Ac | 26.911±0.244Ad | 39.6±0.480Ac |
| Midgut | 0.003±0.002Ba | 58.375±5.240Bbc | 52.583±0.751Bc | 67.371±1.162Bb | 30.66±3.440Bd | 43.541±2.760Bdc | |
Table 1: The effect of the different concentrations of E. coli on the protein carbonyls amount (OD/μg protein) and lipid peroxides concentration (mM cumene hydroperoxides/ μg protein) in thoracic muscles and whole midgut homogenates of 5th instar S. gregaria.
Injection of E. coli into the hemocoel of the 5th instar S. gregaria caused a significant increase in protein carbonyls amount, 1 h post injection (P.I.) of this stressor was about 7% (t=11.105; p< 0.0001) and 31% (t=19.181; p< 0.0001) with respect to control in thoracic muscles and whole midgut, respectively(Figure 1a).
Figure 1: Concentrations of protein carbonyls (a), and Concentrations of lipid peroxides (b) in thoracic muscles and whole midgut homogenates of 1-day starved 5th instar S. gregaria post injection with 15 μl of 12 cell heat killed E. coli per individual. Values are mean±S.E. (n=3). Bars marked with different capital letters indicate statistical significance between thoracic muscles and midgut (independent t-test; p<0.05) and small letters indicate statistical significance among experimental times (one-way ANOVA; p< 0.05).
The protein carbonyls levels were increased up to 178% and 192% with respect to the control value in thoracic muscles and midgut, respectively.
Lipid peroxides: A significant increase in lipid peroxides concentration were observed as a result of the injection of 12 cell E. coli/15 μl saline/ individual into the hemocoel of the 5th instar S. gregaria at 1 h P.I. of this stressor. The percentage increase lipid peroxides concentration was about 87% (t=18.016; p<0.0001) and 1135% (t=16.602; p<0.0001) with respect to control value in both of thoracic muscles and midgut, respectively (Figure 1b).
DNA strand breaks: DNA damage (strand breaks) were measured using comet analysis, as tail moment values (tail length×% DNA in tail), as well as values of the % severed cells. After 24 h P.I. (a representative test-time) of heat killed E. coli treatments, Tail moment value increase about 45% and 31% in thoracic muscles and midgut cells, respectively with respect to control value (Table 2), The number of severe damaged cells, the % values of these cells were increased by 16% and 15%, in thoracic muscles and midgut, respectively, in comparison to control (Table 2 ).
| Comet Length(PX) | Comet Height(PX) | Comet Area(PX) | Head Intensity(PX) | %DNA in Head | Tail Length (PX) | Tail Area (PX) | Tail Intensity | %DNA in Tail | Tail moment | %of severed cells | Tissue | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51.46±47.33aA | 52.62±47.09aA | 4123.10±990.21aA | 98.08±4.47aA | 98.08±4.47aA | 1.28±0.41aA | 33.90±132.21aA | 4525.52±831.13aA | 1.91±0.47aA | 0.03±0.01aA | 6%aA | Muscle | Control |
| 46.28±39.29aB | 4798±38.88aB | 2989.20±849.91aB | 439345.96±1.27aB | 98.39±2.45aA | 1.08±0.46aA | 47.60±39.76aB | 7238.56±470.19aB | 1.60±0.45aA | 0.02±0.01aA | 10%aB | Midgut | |
| 52.01±40.50aA | 51.62±45.09aA | 4223.10±90.21aA | 99.08±4.67aA | 99.08±4.47aA | 1.38±0.51aA | 35.90±22.21aA | 4825.52±831.13aA | 1.81±0.47aA | 0.05±0.01aA | 6%aA | Muscle | Saline |
| 45.63±40.89aB | 48.98±38.88aB | 2999.20±849.91aB | 459345.96±1.27aB | 99.39±2.45aA | 1.28±0.46aA | 47.60±39.76aB | 7738.56±470.19aB | 1.60±0.45aA | 0.02±0.05aA | 10%aB | Midgut | |
| 40.59±38.51bA | 43.98±31.8bA | 3896.58±415.89bA | 50.12±36.87bA | 93.89±3.50bA | 2.94±0.71bA | 70.69±48.92bA | 4835.89±351.87bA | 7.50±13.80bA | 0.48±0.14bA | 22%bA | Muscle | E.coli |
| 36.56±27.20bB | 38.68±28.9bB | 1387.34±53.56bB | 98646.00±4.3bB | 92.36±14.2bA | 2.20±1.90bB | 52.14±4.63bB | 7683.90±743.75bB | 8.75±5.74bB | 0.33±0.01bB | 25%bB | Midgut |
Table 2: Analysis of DNA damage using alkaline comet assay (pH≥13.0) in thoracic muscles and midgut cells of 1-day starved 5th instar S. gregaria injected with 15μl of 12 cell heat killed E. coli per individual and assayed at 24 h post injection (recommended time). Each replicate=50 cells. Raw in the same column marked with different letters indicate statistical significance (p<0.05) between thoracic muscles and midgut cells (capital letters) was executed by independent t-test and between treatments (small letters) was executed by one way.
Activity of antioxidant enzymes
The effects of different concentrations of (0-36 cell E. coli/ 15μl saline/individual) on the activities of antioxidant enzymes SOD, CAT, GPx, and GR were determined (Table 3).
| GR (OD/µgprotein/min) | GPx (OD/µgprotein/min) | CAT (OD/µgprotein/min) | SOD (OD/µgprotein/min) | E.coli concentration (cell/15µl) | ||||
|---|---|---|---|---|---|---|---|---|
| Midgut | Muscle | Midgut | Muscle | Midgut | Muscle | Midgut | Muscle | |
| 0.264±0.017Ba | 0.131±0.006Aa | 0.373±0.026Ba | 0.094±0.006Aa | 0.423±0.003Ba | 0.587±0.002Aa | 0.086±0.002Ba | 0.069±0.001Aa | Control |
| 0.670±0.025Bb | 0.143±0.005Aa | 0.538±0.038Bb | 0.073±0.001Aa | 4.257±0.253Bb | 1.964±0.034Ab | 0.299±0.003Be | 0.025±0.002Ab | 6 |
| 0.748±0.017Bbc | 0.256±0.018Ab | 0.548±0.022Bb | 0.088±0.004Aa | 5.493±0.112Bc | 2.313±0.112Ab | 0.307±0.007Bbc | 0.138±0.001Ac | 12 |
| 1.138±0.003Bd | 0.594±0.045Aa | 0.634±0.003Bb | 0.176±0.009Ab | 6.611±0.235Bd | 2.478±0.218Ab | 0.369±0.014Bd | 0.338±0.015Ad | 24 |
| 0.720±0.039Bb | 0.101±0.001Aa | 0.383±0.021Ba | 0.090±0.001Aa | 3.738±0.051Bb | 2.350±0.063Ab | 0.263±0.020Bc | 0.047±0.002Aa | 30 |
| 0.850±0.026Bc | 0.121±0.001Aa | 0.603±0.012Bb | 0.096±0.001Aa | 4.229±0.201Bb | 2.251±0.092Ab | 0.354±0.004Bdc | 0.116±0.001Ac | 36 |
Table 3: The effect of the different concentrations of E. coli on the activity of antioxidant enzymes in thoracic muscles and whole midgut homogenates of the 5th instar S. gregaria.
The results showed constitutive activity levels of the antioxidant enzymes SOD, CAT, GPx, and GR, in the supernatant tissuehomogenate extract of thoracic muscles and midgut homogenates of the 1-day starved 5th instar S. gregaria .
The partial characterization of these enzymes showed that the optimal pH values were 10.0 for SOD and 7.0 for CAT, GPx, and GR (data not shown). The optimal enzymes concentration were 100% for SOD, GPx, and GR and 25% for CAT The optimal time of incubation of enzymes were 4 minutes for SOD, GPx, and GR and 1 minutes for CAT (Figure 2-4).
Figure 2: A photomicrograph of DNA damage, as revealed by the alkaline comet assay (pH≥13.0), in thoracic muscles and whole midgut homogenates of 1-day starved 5th instar of S. gregaria assayed at 24 hour post injection (recommended time), control (A, B), 15 μl of saline (C, D), 15 μl of 12 cell heat-killed E. coli (E, F) per individual. 50 cells were analyzed per sample (50 cells per slide and 3 slides per treatment were assessed). Scale bar represented as 75 μm.
Also, the results showed that injection with the stressor, heat killed gram positive E.coli, led to increase the activities of the four antioxidant enzymes; with respect to those of the constitutive levels; this begin from the first hour P.I. (Figure 5).
Figure 5: Activity of the antioxidant enzymes SOD, CAT, GPx, and GR, compared to control, and expressed as OD480, 240, 420, 420 respectively/μg protein/minutes in the thoracic muscles and whole midgut homogenates of 1-day starved 5th instar S. gregaria measured at different time intervals post injection with 15μl 12 cell heat killed E.coli. Values are mean±S.E. (n=3). Bars marked with different capital letters indicate statistical significance between thoracic muscles and midgut (independent t-test; p<0.05) and small letters indicate statistical significance among experimental times (one-way ANOVA; p<0.05).
The time course differences in the inducible levels of activity of the antioxidant enzymes due to injection of the stressor had no certain patterns, but they varied throughout the time course of the experiment (Figure 6).
The activities of both the constitutive and induced level of GPx and GR were always increase in whole midgut homogenate than in thoracic muscle of S. gregaria; however, this difference was not always present in the case of SOD and CAT (Figure 6).
Reactive oxygen species (ROS) are generated by all organisms during metabolic processes occurring under aerobic conditions. Their quantity increases as an effect of both abiotic (radiation, climatic factors) and biotic stressors. That later group includes pathogenic factors such as viruses, bacteria and parasites [27].
The harmful effects of the indirect stressor heat killed bacteria E.coli were assessed in vivo in thoracic muscles and whole midgut of the 5th instar S. gregaria after their injection into the hemocoel of this insect. The stressor used in the present work represent an indirect source of reactive nitrogen species (RNS) such as nitric oxide radical (NO•), as a result of innate immune response of insect to challenging with bacteria [11]. These ROS and oxidative stress can be assessed indirectly by assaying augmented oxidative damage to the macromolecules proteins, lipids, and DNA as well as elevated activities of antioxidant enzymes [8,9] as conducted in tissue homogenate of thoracic muscles and whole midgut and of 1-day starved nymphs S. gregaria.
Under in vivo conditions, multiple factors may interfere in the reactions of these stressors, not only, formed oxidative damages, and oxidants roles. But also, elevated expression levels of four principal antioxidant enzymes, SOD, CAT, GPx, and GR [8,12], in the thoracic muscles and whole midgut tissues of S. gregaria in response to produced ROS, were assessed.
The injection of 12 cell of this stressor per individual nymph S. gregaria, results in oxidative damage to proteins, lipids, and DNA from the 1st h P.I. with significant difference from that of controls. These measured damages were in the form of protein carbonyls, lipid peroxides, and DNA strand breaks, respectively, as compared to controls. The observations started from the 1st h P.I. and extended throughout the time of the experiment, 72 h P.I. for proteins and lipids (but for DNA, it was observed only once after 24 h P.I.).
For the protein carbonyls, ROS can cause reversible oxidations to some sulfur-containing protein-amino acids; therefore, these oxidized proteins are repairable. Also, irreversible oxidations of proteins produce both protein-sulfonic acids as well as the only indicated and measured protein carbonyls in S. gregaria (Figure 1) which are not repairable, and should be degraded [28]. As protein carbonyls are formed from oxidation of specific amino acids (arginine, histidine, lysine, and proline) and polypeptide chains cleavage (at aspartate, glutamate, and proline) [28,29] .
The oxidation of proteins leads to disruption of conformation and vital functions of protein molecules, including enzymes, and other regulatory functions of the cell [10,12]The factors that may control protein carbonyls may be genetic and lysosomal-mediated cellular proteolytic processes [28,30-34]. Therefore, the observed fluctuations in amount of protein carbonyls shown throughout the time-course changes in S. gregaria (Figure 1) may reflect fluctuating homeostatic mechanisms between production of protein carbonyls and their degradation.
Lipid peoxides may be initiated by OH, or any other reactive free radical, by abstracting a hydrogen atom from the unsaturated fatty acid [10,35,30]. Therefore, lipids peroxidation processes can result in formation and elevation of the determined lipid peroxides concentration in the S. gregaria (Figure 2). At the same time, the terminating and repairing processes are able to minimize the concentrations of lipid peroxides (Figure 1b). These may not only, include recombination of lipid peroxyl radicals, but also include their reaction with glutathione in a GPx-catalyzed reaction [30,35]. Hence, the fluctuating pattern in the determined concentrations throughout the time course P.I. implies to reflect the resultant of these homeostatic mechanisms. Lipids peroxidation was informed that biologically disrupt structure and function of membrane polyunsaturatedphospholipids bilayer [36]. Products of lipid peroxidation are also lead to disrupt conformations of many cellular proteins, including enzymes, by forming cross links with these proteins. This occurred direct and indirect way, as well as the mentioned direct, damaging processes leads to inhibition of cell functions [12].
For DNA single strand breaks, the values of tail moment, as an random expression for the quantitative estimation of DNA strand breaks [37], in the case of injection of heat killed E. coli, shows that this stressor has led to DNA strand breaks in cells of thoracic muscles and midgut of S. gregaria (Figure 2 and Table 2). Also, the percentage severed cells (Table 2) used as a possible supplementary criterion [38]. The results on DNA oxidative damage (Table 2) suggest that this stressor is able to produce oxidative damaging stress.
Antioxidant enzymes response to oxidative stress
Antioxidants, involving nonenzymatic and enzymatic antioxidants, can restored homeostasis between oxidants and antioxidants [3,12,39-41]. The results showed a low level of constitutive activity in the control samples of each of the four essential antioxidant enzymes concerned in the present work SOD, CAT, GPx, and GR, in the tissuehomogenate extract of the whole midgut and the thoracic muscles of 1- day starved 5th instar S. gregaria. These antioxidant enzymes catalyze reactions presented in equations 1-5 [15,3,6,18,42-47].
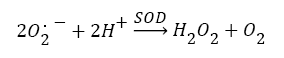 (1)
(1)
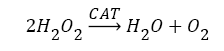 (2)
(2)
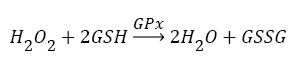 (3)
(3)
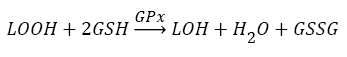 (4)
(4)
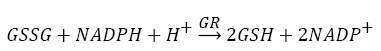 (5)
(5)
From the partial characterization of the enzymes determined in S.gregaria, values of Km , pH optima, incubation time, and enzymes concentrations were identified (Figure 3-6). The results show that injection with the stressor heat killed E. coli has led to increase the activity of the four principle antioxidant enzymes, from the beginning of the first hour P.I., with respect to control levels. The activities of both the constitutive and induced GPx and GR are always higher in whole midgut homogenate than in thoracic muscle of S. gregaria; however, this difference is not always present in the case of SOD and CAT (Figure 6). The increase in the activity of the principle antioxidant enzymes seems to occur in accompaniment with oxidative damages to the macromolecules proteins, lipids, and DNA indicated above. This association may imply that it occurs in response to formed ROS in consequence to the injected stressors. The differences between the induced activities of these four antioxidant enzymes throughout the time course up to 72 h P.I. have no definite pattern (Figure 6). [48], found that bacteria, BT infection resulted in increasing the activities of SOD, GST, on the 1st day after inoculation. However, catalase activity decreased on the first and following days after bacterial infection by Bt. Galleria mellonella larval. These results confirmed the hypothesis that bacterial infection increases the level of oxidative stress in the larval midgut). The formed ROS were cited before [12] involve in a signal transduction pathway affecting regulation of antioxidant genes and induce expression of several genes [49-51]. The present results agreed with [48], who found that bacteria, BT infection resulted in increased activities of SOD, GST, on the 1st day after inoculation. However, catalase activity decreased on the first and following days after bacterial infection by Bt. Galleria mellonella larval. These results confirmed the hypothesis that bacterial infection increases the level of oxidative stress in the larval midgut).
I wish to thank staff members of Entomology Department, Faculty of Science, Cairo University, for their effective guidance, and invaluable assistance.