Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Research Article - (2016) Volume 5, Issue 1
Keywords: Biosensor; Horseradish peroxidase; Hydrogen peroxide; Glutaraldehyde; Polyaniline
Hydrogen peroxide is one of the molecules involved in reactive oxygen species. Hydrogen peroxide are produced by catalysis of glucose oxidase, cholesterol oxidase, xanthine oxidase, alcohol oxidase, and uricase reaction. These enzymes were applied for monitoring levels of glucose, cholesterol, xanthine, alcohol, and gout. The advantage of using enzyme as component of biosensor is high selectivity to interact with specific substrates.
There are several enzymes that can be used for biosensors development such as catalase [1], cholesterol oxidase [2], myoglobin [3], glucose oxidase [4], bilirubin oxidase [5], laccase [6] and others. Horseradish peroxidase (HRP) is one of enzyme that used in electrochemistry as biosensor [7] and biofuel cell [8], as decolorization agent [10], immunoassay [11], biodegradation [12] and synthesis of polyaniline [13]. The horseradish peroxidase (1.11.1.7) is classified as oxidoreductase enzyme that can be used as biosensors. HRP enzyme can be isolated from some organisms such as roots of plant radish, E. coli, mammalian cells and yeast [9]. One of the critical point should be considered to make biosensor is electron transfer. Electron transfer is more efficient by using metal, but the price is more expensive and needed to replace metal. Other compound that can be used for electron transfer is polyaniline. Polyaniline has unique properties was called doping dedoping or protonation deprotonation [14].
Polyaniline (PANI) is a polymer with conductive properties and has a role during electron transfer. PANI also can be used as matrix for enzyme immobilization in order to provide stability of enzyme more stable as a biosensor. PANI has good conductivity properties and has potential to be developed in the field electrochemistry. PANI has been used to produce biofuel cell [15] and biosensor [16] as conductor during electron transfer. PANI has been chosen as conductor based on high conductivity and can be synthesized easily and inexpensive by using interfacial method [14]. Also, PANI has ability to change the electrical and optical properties that can be reversible through redox reactions and protonation-deprotonation.
The high performance of HRP and PANI as biosensor was influenced by electrochemical properties during transfer electrons between electrode and active site of enzyme [17]. The aim of this study was to develop biosensor hydrogen peroxide by using horseradish peroxidase that immobilized with glutaraldehyde cross linking at carbon PANI nanofiber composite. Chitosan is one of immobilization agents used to develop biosensor hydrogen peroxide. Yanciner et al. [18] have examined biosensor hydrogen peroxide by used Nickel Ferrite Nanoparticle-Chitosan Composite as immobilization agent by cross-linking methods. Glutaraldehyde and chitosan have same characteristic. They have two functions consist of as crosslinker and surface activating agent [19]. In this study, we used glutaraldehyde which modified by PANI nanofiber as crosslinker agent.
The materials were used in this study are potassium chloride, distilled water, nanofiber PANI, the enzyme horseradish peroxidase (Sigma), glutaraldehyde, bovine serum albumin (BSA), phosphate buffer, graphite, paraffin, K3Fe(CN)6, K4Fe(CN)6, copper wire, tube Teflon and glass tubes. The Tools were used include glass tools, eDAQ potentiostat-galvanostat which includes software Echem v2.1.0.
Fabrication of electrode
The fabrication of carbon paste electrodes (CPE) was prepared by mixing 0.15 gr of graphite and 100 mL of paraffin with mortal for 30 minutes to homogenize carbon paste. A glass tube as electrode (diameter of 0.8 cm and 3 cm length) filled with carbon paste and connected with copper wire for electric source and electrode. The modified carbon paste electrodes (MCPE) PANI were prepared with 0.15 gr mixed carbon and 100 mL of paraffin with 2 mg PANI in mortal (Figure 1). This electrode was stored in refrigerated condition.
The CPE and MCPE were prepared based on Colak et al. [20]. Nanofiber PANI was prepared by Maddu et al. [14] with interfacial method.
Preparation of HRP/GA/PANI electrode
Immobilized HRP was done as referred to Yang et al. [21] by cross linking method using glutaraldehyde. Immobilization of enzyme was prepared by mixing of 20 μL of HRP (5000 U/mL), 0.4 mg of bovine serum albumin (BSA), 10 μL of glutaraldehyde (2.5% w/v) and 20 μL of phosphate buffer at pH variation (pH 6-8, with range of 0.5). The amount of 50 μL of the mixtures was pipetted onto surface of modified carbon paste electrodes (MCPE). The electrode was dried at 4°C until the solution of immobilized enzyme adsorbed in the carbon-nanoparticle PANI paste. Then HRP/GA/ PANI electrodes was washed by using distilled water to determine the amount of the immobilized enzyme.
Optimization and characterization of electrode
Carbon paste electrode (CPE), modified carbon paste electrode (MCPE) and HRP/GA/PANI were characterized by using cyclic voltammetry in 0.1 mM of phosphate buffer (from pH 6-8 at an interval of pH 0.5) containing 0.1 M K3Fe(CN)6/K4Fe(CN)6 (1:1). The optimum temperature was determined by incubating the reaction mixture at different temperature (30-80°C at an interval of 5°C). Hydrogen peroxide concentration was prepared in reaction buffer in the range 0.1-0.8 mM to obtain Km and Imax values as kinetic parameters that performed by using cyclic voltammetry. Variations concentrations were tested in the range of 0.1 mM-0.8 mM at optimum pH 7.0.
Cyclic voltammograms carbon pasta electrode (CPE) and modified carbon pasta electrode (MCPE)
The cyclic voltammogram of carbon paste electrodes (CPE) and modified carbon paste electrodes (MCPE) was determined to compare performance of electrodes based on reduction oxidation reactions that occurred at electrode surface. Modification of electrodes was made with the addition of PANI to enhance the electron transfer. As shown at Figure 2, addition of PANI could enhance electron transfer by increasing reduction peak compared with CPE without the addition of PANI.
Performance MCPE during electron transfer is higher than CPE without PANI (Figure 2). CPE electrode had lower reduction peak than the MCPE. It explained that MCPE electrode could produce electric current higher than CPE electrode. The electric current increased due to modifications of electrode with addition of PANI nanofiber which have conductive properties. The electrode performance was also influenced by surface area of electrode. Reaction between electrodes and electrolyte solution occurred depend on the surface area [22]. Carbon have smaller surface area, which only reached 16.30 m2/g, while surface area of the carbon paste electrodes with PANI modifications reached 29.26 m2/g [23]. Therefore, MCPE electrode was more effective than CPE on the electron transfer process, so that it can be used as electrode development.
Peak current at CPE electrode oxidation were resulted at 0.0851 mA at a voltage 0.108 V, there is no formation of peak reduction. The reduction current of CPE was smaller than MCPE with value 0.2306 at voltage 0.188 V. Current formation was influenced by conductive polymers (nanofiber PANI) and electrode preparation that effect electron transfer.
The optimum pH of HRP/GA/PANI electrode
The effect of pH on performance of H2O2 biosensor was indicated by peak that described reaction of oxidation and reduction. Reduction of H2O2 to H2O was indicated as reduction peak. If performance of electrodes is high, greater peak will be resulted in cyclic voltammetry. Optimum pH produced highest electric current at pH 7.0 as showed in cyclic voltammogram (Figure 3). At the optimum pH 7.0, the biosensor produced 0.969 mA for reduction peak at potential-0.614 V. The highest of electric current produced at pH 7.0 and indicated optimum performance of the HRP/GA/PANI electrode. Meanwhile, there is no electric current formation at pH 6.0, 6.5, 7.5 and 8.0 which indicated oxidation and reduction reactions (Figure 4).
Horseradish peroxidase enzyme was immobilized before entrapment into electrode with glutaraldehyde. HRP was immobilized by cross-linking for stability enzyme at certain conditions in electrode. Immobilization can enhance stability of enzyme as biosensor.
The influence of pH on HRP/GA/PANI performance electrode has been investigated by cyclic voltammetry of 0.5 mM H2O2 in buffer phosphate 0.1 M and K3Fe(CN)6:K4Fe(CN)6 (1:1) 0.1 M at different pH value between 6 and 8 (range 0.5) (Figure 3). The electric current of HRP/GA/PANI electrode increased as increasing of acidity value, but electric current value declined sharply over pH 7.0 (Figure 4). The value of reduction current at optimum pH 7.0 was 0.96 mA at -0.168 V.
Based on Chang and Tang [24], non-immobilized enzyme (free enzyme) has lower optimum pH compared to immobilized enzyme. The difference of optimum pH was caused by changes of enzyme conformation that effect binding capacity of enzyme to substrate. Temocin and Yigitoglu also found that optimum pH of HRP immobilized was pH 7.0 [25].
Optimum temperature HRP/GA/PANI electrode
The highest electric current was produced at temperature 50°C as shown in cyclic voltammogram (Figure 5) The HRP/GA/PANI electrode had optimum temperature at 50°C (Figure 6) that produced reduction current 4.972 mA and 0.63 V. Optimum temperature of HRP/GA/PANI electrode was higher compare to Chang and Then that found for HRP was 40°C [24]. Electric current decreased at above 50°C that indicated horseradish peroxidase was denatured.
The surface concentration (Γ) of HRP/GA/PANI electrode has been determined by used Brown-Anson model (1):
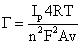
The surface concentration of HRP/GA/PANI electrode has been calculated using Brown-Anson (1). Ip is value of peak reduction, n is the number of electrons transferred, F is the Faraday constant (96485 C/mol), Γ is the surface concentration of HRP/GA/PANI (mol/cm2), A is the surface area of electrode (cm2), V is the scan rate (mV/s), R is the gas constant (8.314 J/molK), and T is the temperature (K). The surface concentration of HRP/GA/PANI electrode has been determined as 7.136 × 10-9 mol/cm2 was higher than of HRP-PANI-ClO4/ITO electrode as 5.81 × 10-9 mol/cm2 [17].
Kinetic parameters of HRP
Kinetic parameters of horseradish peroxidase was determined to characterize electrode as biosensor based on cyclic voltammogram. Determination of the kinetics parameter were obtained Km and Imax values performed using cyclic voltammetry method. Variations concentrations were tested in the range of 0.1 mM-0.8 mM under optimum pH (pH 7). Kinetic parameters of horseradish peroxidase was showed in Figure 7. The electric current value was plotted as Michaelis- Menten curves (Figure 8).
The linearity of curve between substrate (hydrogen peroxide) and current value of HRP/GA/PANI electrode which obtained at linear region of Michaelis-Menten curves (Figure 8). At concentration of 0.1 mM of hydrogen peroxide produced electric current and concentration of hydrogen peroxide increased simultaneously. However, at concentration higher than 0.5 mM of hydrogen peroxide did not increase electric current significantly due to HRP enzyme already saturated. The linearity region of HRP/GA/PANI electrode at concentration 0.1-0.3 mM by R2 was 0.9947 (Figure 9). The kinetic parameters consisted of Km and Imax values were 1.71 mM and 0.29 mA, respectively and sensitivity of HRP/GA/PANI electrode was 12.14 mA/mMcm2.
Biosensor performance parameters of HRP/GA/PANI electrode was investigated by using cyclic voltammetry method in K3Fe(CN)6:K4Fe(CN)6 0.1 M (1:1) and 0.1 M phosphate buffer and H2O2 as a substance to be reduced. The effect of performance of HRP/GA/PANI to H2O2 concentration was showed in Figure 8. The performance activity of HRP/GA/PANI electrode was examined by the current produced as a product. The result of it curves the Km and Imax was calculated as 1.71 mM and 0.29 mA, respectively.
The electric current value presented reaction rate of catalysis HRP to H2O2. The formation of enzyme-substrate complexes was determined based on rate of reaction. Therefore, the reaction rate and product formation was influenced by formation of enzyme-substrate complexes. The reaction rate is higher when concentration of substrate was low and this condition produced linearity between substrate and product. Thus, the product of catalysis did not increase significantly at high substrate concentration that found state of enzyme-substrate complex as known maximum reaction rate (Vmax). The electric current was not produced at concentration of 0.5 mM. It indicated the enzyme was saturated at maximum of reaction rate. Compared to previous studies, the Km value in this research was slightly lower than Solanki [17] with the value of Km was 1.984 mM. This result confirmed that HRP/GA/PANI electrode can be further developed as biosensors (Table 1).
| Parameter | Optimum condition |
|---|---|
| Temperature | 50°C |
| pH | 7.0 |
| Km and Imax | 1.71 mM and 0.29 mA |
| Linearity | 0.1-0.3 mM (R2=0.9947) |
| Sensitivity | 12.14mA/mMcm2 |
| Surface Concentration | 7.136 × 10-9 mol/cm2 |
Table 1: Optimum condition of HRP/GA/PANI electrode.
Characterization of biosensor for hydrogen peroxide by using horseradish peroxidase (HRP) enzyme has been done. The HRP was immobilized by cross linking with glutaraldehyde onto carbonnanofiber PANI composite. The presence of nanofiber PANI improved performance of HRP/GA/PANI electrode as biosensor. Optimum performance of HRP/GA/PANI electrode was at pH 7.0 and 50°C. Based on the Michaelis-Menten curves, the performance of HRP/GA/ PANI electrode are in the linear region at 0.1-0.3 mM [Figure 10]. The Km and Imax value of HRP/GA/PANI electrode were 1.71 mM and 0.29 mA, respectively. Biosensor of hydrogen peroxide by using HRP/GA/ PANI has good response with sensitivity value of 12.14 mA/mMcm2 and surface concentration was 7.136 × 10-9 mol/cm2.
We would like to say thank to Directorate General of Higher Education, Republic of Indonesia (DIKTI) for funding this research as “Postgraduate Education Scholarships”.