Journal of Medical Diagnostic Methods
Open Access
ISSN: 2168-9784
ISSN: 2168-9784
Research Article - (2016) Volume 5, Issue 1
This exploratory, descriptive study aims to explore general practitioners’ viewpoint about biosimilar drugs, conducted on a sample of 128 randomly selected general practitioners serving in public sector hospitals in Karachi, Pakistan. They were surveyed with a 12 items questionnaire that evaluates their approach towards biosimilar drugs. The collected data was analyzed for frequency distributions and χ2 using SPSS. The present findings highlight a need for further education of physicians and others related to the prescribing of biosimilar medicines as poor knowledge could result in serious medication errors, adverse events or a delay in desired therapeutic gain for the patient. Further dialogue and collaboration between physicians, authorities and healthcare biotech industry should be continued on a priority basis.
Keywords: Biosimilar drugs; General practitioners; Healthcare biotech industry; Pakistan
Biotech drugs are an essential part of modern drug therapy and expected to reach a share of 50% in the pharmaceutical market in the coming years. TechNavio’s analysts forecast the global biosimilar (BSM) market to grow at a compound annual growth rate (CAGR) of 27.58% over the period 2013-2018 [1]. It is four times faster than the small molecule growing market, and is expected that biopharmaceuticals to represent 30% of all drugs marketed in the next five years.
Twelve biological products with global sales of more than US$ 67 billion will be exposed to BSM competition by 2020 [2].
BSMs or follow-on biologics (FOBs) are biological products which are replicas of innovative biopharmaceuticals. BSMs put effort to reproduce the original technology leading to the production of innovative biotech medicines for a product similar to the original. Due to ease of access and affordability, BSMs have been recognized a good standing amongst healthcare experts [3]. Although BSMs have marked recognition in nationalized and global markets, it is vital to consider that the BSMs are not biological generics. These are rather distinctive molecules which are maintained by only narrow clinical data at the moment of approval [4]. Unlike chemical generics, the BSMS require stricter criteria for the evaluation of the quality, safety and efficacy.
Negotiations regarding BSMs began (late 1990s) with patent expiry events of several best-sellers biopharmaceutical drugs which were imminent. In 1984 it was comprehend that the Hatch-Waxman Act or Drug Price Competition and Patent Term Restoration Act [5], the legislation that directs the expansion and commercialization of generic versions of small molecule drugs, did not give a legal regulatory structure for the approval of this class of molecules in the US. Similarly, there was no regulatory direction for approval in Europe or somewhere else in the world. This marked the commencement of long discussions about whether or not BSMs have to be made commercially accessible. Not astonishingly, innovators with approved biopharmaceutical products previously on the market persistently opposed any legislation for BSMs while small molecule generic manufacturers were in support of it. Finally in 2005, the European Medicines Agency (EMEA) structured regulatory supervision that allowed for marketing approval of BSMs in the European Union (EU). Since January 2006, EMEA has granted marketing authorization for 18 new BSM products marking the beginning of a new era in the biopharmaceutical industry. In February 2006, EMEA released guiding principles enclosing particulars of clinical, nonclinical and quality expectations for BSMs [6].
Being a novel field based on an innovative regulatory path, BSMs are in straight antagonism with a number of well-established pacesetter companies with huge budgets. Furthermore, the progress of secondgeneration biopharmaceutical products in the market possibly with improved safety and efficacy than innovative first-generation products is another time a challenge for BSM marketing [7]. BSMs will only be comparable but not identical to the product they look for duplicate. Owing to the fact that proteins are large, complex molecules with innate variability which cannot be entirely controlled throughout the manufacturing process. In biotech medicine, every product has a distinctive safety profile dependent on its mechanism of action, manufacturing process, and composition. Preceding studies have demonstrated the dissimilarities among the bioactivity of the BSMs and their innovator products. Consequently, there are concerns about their efficacy, long-term safety and immunogenicity [6]. As this field continues to evolve and more BSMs are expected to become available in the near future, physicians will need to make informed decisions in the clinical use of BSMs to ensure that high-quality, safe, and affordable drugs are accessible to patients. It is important to ascertain where physicians currently rate their information about BSMs. Poor knowledge of BSM medications could result in serious medication errors, adverse events or a delay in desired therapeutic gain for the patient. Pharmaceutical education of healthcare providers is paramount to ensure patient safety as BSMs are introduced into clinical practice. With this background the current study was conducted to evaluate the awareness of physicians about BSMs.
This exploratory, descriptive study was conducted from June to Oct 2015. The study population comprised of general practitioners who were rendering their services at public sector hospitals in Karachi, Pakistan. They were selected randomly for the study and were surveyed with a 12 items questionnaire that evaluates their approach towards BSM drugs. Questionnaire items solicited data on participants’ socio demographic characteristics (age, gender, position, organization, year of experience, patients per week). Each questionnaire was accompanied by a cover letter explaining the purpose of the study and providing specific instructions for questionnaire completion. Standard procedures of informed consent were used, including the protection of participants’ anonymity and confidentiality. The collected data were entered into Statistical Package for Social Sciences (SPSS), version 20.0 and were analyzed for frequency distributions and χ2 at p < 0.05 significant level.
The response rate of the study was 51.2%. Majority of the respondents were male (59.38%) while 40.63% were female. Majority of the participants were designated as RMOs (35.94%). Forty six % have an experience of less than 5 years. Around 84% were delivering their services in clinical site. More than 46% see 50-100 patients per week. More than half (52.3%) opined that their 50-75% patients requires treatment with biological drugs. An approved BSM is expected to have the same efficacy and safety as the reference biological, but may not necessarily be authorized for all indications approved for its reference medicinal product. A clear understanding of the scientific basis of the BSM concept and access to unbiased information about BSM license is important for physicians to inform and to make appropriate treatment options for their patients. However on inquiring about the familiarity with BSMs, 42.18% stated ‘heard of them-cannot define’ whereas 35.93% never heard of BSMs. (Figure 1) Ildar Akhmetov suggested low to medium levels of BSM awareness among practitioners [8] (Table 1).
| Characteristics | Percentages |
|---|---|
| Gender | |
| Male | 59.38 |
| Female | 40.63 |
| Age (years) | |
| 25-30 | 39.06 |
| 31-35 | 33.59 |
| 36-40 | 21.09 |
| 41-50 | 3.91 |
| 51 and above | 2.34 |
| Position | |
| Consultant/surgeon | 12.5 |
| Chief medical officer | 27.34 |
| Head of department | 10.16 |
| RMO | 35.94 |
| Professor | 5.47 |
| Lecturer | 8.59 |
| Experience | |
| Less than 5 years | 46.09 |
| 5-10 years | 30.47 |
| 10-15 years | 16.41 |
| 15-20 years | 4.69 |
| 20 and above | 2.34 |
| Field | |
| Clinical | 83.59 |
| Academics | 15.63 |
| Organization | |
| Private | 17.7 |
| Public sector | 84.3 |
Table 1: Characteristics of study population.
Another study conducted in Europe reported that most physicians (46%) responded that they had only a basic understanding of biological medicines, while 43% said they had a complete understanding. Only 1% of all physicians surveyed had never heard of biological medicines, while 11% were not able to define them [9]. The question of how physicians had become familiar with BSMs was answered by only 357 of the 470 recruits (76%). Most of these gained familiarity through attending conferences and seminars (47%), while 35% learnt through self-study, 11% through studies sponsored by BSM companies and the remaining 6% split equally between studies sponsored by innovator companies, clinical trial participation and other routes [9]. In our study, more than half (53.12%) learn about BSMs through medical conferences. Other sources include medical associations (17.18%), hospital formularies (7.03%) and patient group (7.8%) (Figure 2). On asking that if BSMs are made available, what your likelihood to prescribe them is, 45% opined that their probability to prescribe is low. Figure 3 illustrated the main reasons for physicians to prescribe BSMs. High percentage (67.18%) affirmed cost effectiveness to be the main reason of prescribing BSMs. While same safety profile as in original biologics (17.96%) and same efficacy in original biologics (10.93%) were thought to be other main reasons.
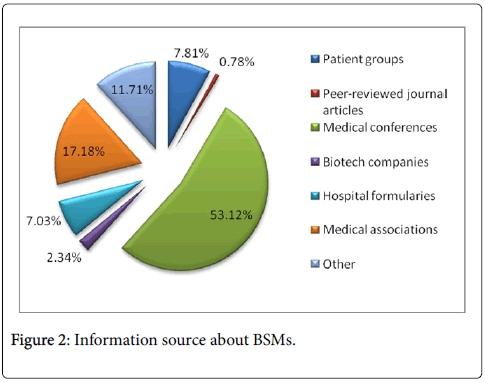
Figure 2: Information source about BSMs.
Many blockbuster biologics worth US$ 50 billion will lose patent protection over the next few years in US alone. As the peak 25 biologics are driving 83% of international sales, patent expiry of these products is building up new potential for BSM players in subsequent years. Advancing disease patterns, product demand, and better tertiary care push enormous business opportunities for companies interested in BSMs. On the other hand, owing to the high clinical development and manufacturing costs, the price difference between BSMs and parallel originator products is still a challenge [10-13]. It needs at least 40-50% price reduction from branded products to meet customer’s expectation [1]. In current study, half of the physicians opined that the price for BSMs should be 40-50% lower in comparison with original drugs. More than 70% did not know that if two medicines have the same nonproprietary scientific name, does this suggest or imply that the medicines are structurally identical. More than 60% did not know if two medicines have the same non-proprietary scientific name, does this suggest or imply that the medicines are approved for the same indication. Around 70% considered it critically important to decide, the most suitable biologic medicine for their disease. When the respondents were asked that in a situation where substitution by a pharmacist was an option in country, how important would it be to you to have the authority to designate a biologic medicine as ‘DISPENSE AS WRITTEN’ or ‘DO NOT SUBSTITUTE’, 72% considered it very important and only 1.6% did not considered it important. The association of the demographic variables on the responses of participants towards questionnaire items was determined by using chi-square at P<0.05 significant level. Significant association was found between age, experience, field, position and organization of respondents on their opinion. However no significant association of gender was observed on responses.
The findings highlight a need for further education of physicians and others related to the prescribing of the BSM medicines. Further dialogue and collaboration between physicians, authorities and the healthcare biotech industry should be continuing on a priority basis.