Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Research Article - (2023)Volume 13, Issue 1
In this study, microbial transformation of monoterpenes (-)-citronellol, (±) citronellene (dihydromyrcene) using Rhizopus oryzae in a Potato Dextrose Broth (PDB) medium was investigated. The major bioconversion product of citronellene was found to be dihydroxycitronellene, which is formed by dihydroxylation of the terminal 6, 7-double bond. On the contrary, (3S)-citronellol yielded 7-hydroxy citronellol as a major product. Several minor products including rose oxide, rose glycol, isopulegol, artemisia-triene, 1, 8-nonan-diol-8-methyl, citronellyl phenyl acetate were obtained from (-)-citronellol. Whereas camphene, camphor, α -pinene, Isopulegone, terpinene-4-ol, and 1, 8-nonan-diol-8-methyl are the minor products obtained from (±) -citronellene. Hence, dihydroxylations and hydroxylation at the selective position of a diene with the help of microbes take place in a single step compared to synthetic routes. Our study emphasizes the role of Rhizopus oryzae in carrying out various kinds of reactions and rearrangement of the molecules which by synthetic route is tedious. The minor products were identified by Gas Chromatography-Mass Spectrometry (GC-MS) analysis and matching fragmentations with the National Institute of Standards and Technology (NIST) library. Some metabolites produced have valuable perfumery application.
Biotransformation; Citronellene; Citronellol; Hydroxylation; Oxidations; Perfumery value
The investigation of new and innovative procedures for obtaining small molecules remains a focus of the pharmaceutical and perfumery industries. Within this context, biotransformation processes offer possibilities for obtaining Regio-selective and stereoselective conversions, which may have industrial importance. Moreover, biotransformation is green processes as they involve very gentle conditions [1]. The transformation of organic compounds by microbial cultures has long been of interest to the pharmaceutical, chemical and food industries because of numerous advantages as compared to chemical synthesis. Fungi consist of a multi-enzymatic system with broad specificities that stand as an alternative to conventional chemical methods. Therefore, it tends to offer various reactions on non-activated molecular sites that are either difficult to react with or unreactive [2]. For the pharmaceutical industry, bioconversion acts as an alternate method for the production of potent molecules this overcomes the problems associated with chemical synthesis and multistep synthesis [3]. In addition, it is also noted that the stereo selectivity of compounds can also be controlled by adjusting the specific condition one of which is pH [4]. Food industry residues consist of lignocellulosic materials recalcitrant towards degradation containing polyphenolic compounds. These can be extracted using biocatalysts as fungal state solid treatment, and enzymes through the process of biotransformation [5]. Biotransformation of monoterpenes has been widely investigated using bacteria, fungi, yeasts, plants or their isolated enzymes [6]. Examples include linalool, limonene, cineole, α -pinene, myrcene, etc. which were converted to their respective epoxy, hydroxyl and further oxidized derivatives such as aldehydes, ketones, carboxylic acids or too-degraded molecules [7,8]. Oxidation of acyclic monoterpenes by cytochrome P450 mono-oxygenase from Bacillus megaterium has been evaluated [9]. Oxidized terpenes and terpenoids are highly valuable compounds for organic chemistry, olfactory value and insect pheromone properties.
Rhizopus oryzae is a filamentous heterothallic micro-fungus that occurs in soil, dung, and rotting vegetation [10]. R. oryzae is used economically in the production of the enzymes, glucoamylase and lipase, in the synthesis of organic acids and in the preparation of various fermented foods [11]. R. oryzae is also known to carry out hydroxylation at saturated and unsaturated carbon centres, epoxidation, ester hydrolysis, sulphide oxidation, and other biodegradation reactions. The expression of a fungal α -steroid hydroxylase from R. oryzae, which can be used to perform the α -hydroxylation of the steroid skeleton, has resulted in a significant reduction in the cost of corticosteroid drugs [12].
The products obtained are thus, natural metabolites having applications in various fields. 7-hydroxy citronellol, dihydroxy citronellene and rose oxide are used as flavours as well as fragrances in the formulation of perfumes, cosmetics, antiseptics etc. Isopulegol is a fine chemical widely employed in pharmaceuticals, agrochemicals, cosmetics, toothpaste, etc. Also, isopulegol acts as a potential therapeutic drug against the clinical completion of Parkinson’s disease [13]. α -Pinene also possesses the therapeutic potential and is a great ingredient in essential oil for formulating cosmetics and perfumes. Terpinene-4-ol is a main bioactive component of tea tree oil that has been shown to have many biological activities [14,15].
In the present work, fungal biotransformation of citronellol and citronellene yielded high-value fragrances compounds such as 7-hydroxy citronellol and dihydroxy citronellene respectively as major products. To the best of our knowledge, there are no other fungi that have yet been reported to carry out the conversion of citronellene to dihydroxycitronellene in a single step with the help of R. oryzae.
In this paper, we report the biotransformation conditions and the identifications of structures of products from the bioconversion of citronellol and citronellene using R. oryzae.
The reaction progress was monitored by Gas Chromatography (GC) and Thin Layer Chromatography (TLC) analyses. TLC was performed on Merck silica gel plates 60 F254 and visualized using anisaldehyde/sulfuric acid reagent or ultraviolet absorption. Gas Chromatography Mass Spectrometry (GC-MS) analysis was carried out on Agilent Intuvo 9000 instrument in the DB-5 column. The chiral Gas Chromatography (GC) analysis was obtained using chiral Beta-dex 120 columns (30 m × 0.25 mm). Infrared spectroscopy (IR) spectra (in CHCl3 or neat) were recorded on a Fourier transform infrared spectrometer. The fungus Rhizopus oryzae, NCIM 1009, (ATCC 9363) culture was obtained from National Chemical Laboratory, Pune, India, and culture media were obtained from Himedia, Mumbai, India. The cultures were grown aerobically at 27°C and 150 rpm on an orbital shaker (Scigenics Biotech, Orbiter). Citronellene and (S) citronellol were obtained from S. H. Kelkar Company, Mumbai, India. Solvents were distilled and terpenes were vacuum-distilled before use. Culture media the Potato Dextrose Broth (PDB) containing potatoes infusion of 200 g /L, dextrose 20 g /L, in distilled water (pH 5.1 ± 0.2) was used. The cultures were maintained on Potato Dextrose Agar (PDA) containing potatoes infusions 200 g /L, dextrose 20 g /L, and agar 20 g/L in distilled water (pH 5.1 ± 0.2).
Biotransformation
Rhizopus oryzae was grown on PDA slants. Inoculation was done by transferring a loopful of mycelium into the flask containing Potato Dextrose Broth (PDB) (100 ml) in each flask. Cultures were allowed to grow in 10 flasks aerobically in the medium on an orbital shaker set at 100 rpm-150 rpm and placed in a room maintained at 27°C for 72 hrs. Subsequently, the substrate citronellol and citronellene (100 mg) dissolved in ethanol (1 ml) were added to each flask aseptically and the fermentation was continued for a further 120 hrs with the agitation speed of 130 rpm. The substrates were incubated with R. oryzae for 120 hours on an orbital shaker. To monitor the biological reaction another, set of 10 flasks was accompanied. After every 24 hours, 200 ml of culture from another set was filtered and extracted and the crude product of it was subjected to Gas Chromatography (GC) to analyze the progress of the reaction. The optimum values of pH and temperature for biotransformation were found to be pH 5.5 at 27°C for the mentioned incubation period.
Extraction of products
Extraction of the bioconversion product was carried out after 120 hours for citronellol and 48 hours for citronellene of the fermentation period. The medium was filtered under a vacuum and cells were separated. The broth containing the products was extracted with ethyl acetate (3 ml × 25 ml). The organic layer containing the product was separated from the aqueous layer. The organic layer was dried over anhydrous Na2SO4 for 2 hours. The solvent was removed under reduced pressure to give reaction products.
Purification of products
The reaction products were purified by silica gel column chromatography using a mixture of ethyl acetate: and petroleum ether (60-80°C) with increasing polarity. After elution, the solvent was removed under reduced pressure to give pure molecules, which were identified with spectral analyses including Fourier Transform Infrared (FTIR), 1H and 13C-Nuclear Magnetic Resonance Spectroscopy (NMR), and Gas Chromatography (GC)/Electron Ionization Mass Spectra (EIMS) analysis. The minor products were identified by GC/EIMS fragmentation matching the spectra from the National Institute of Standards and Technology (NIST) library (Figures 1 and 2 and Table 1).
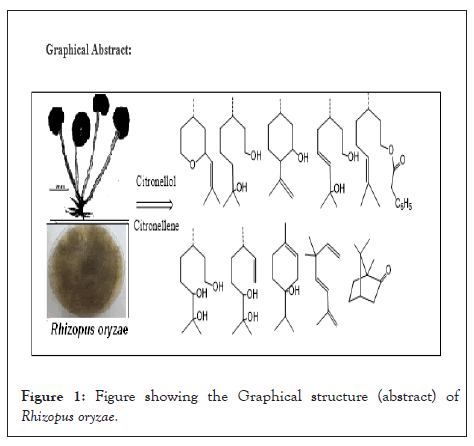
Figure 1: Figure showingtheGraphical structure (abstract) of Rhizopus oryzae.
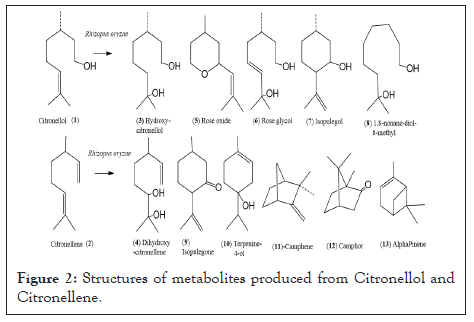
Figure 2: Structures of metabolites produced from Citronellol and Citronellene.
| Substrate | The crude product (g/L) | Products with % yield and (GCMS rt min) | ||||||
|---|---|---|---|---|---|---|---|---|
| Citronellol | 0.6 g | 3 34.30 (9.34) |
5 28.23 (6.54) |
6 10.25 (9.82) |
7 7.28 (10.75) |
8 4.08 (9.3) |
14 8.09 (9.92) |
15 14.89 (21.98) |
| Citronellene | 0.43 g | 4 35.58 (8.76) |
8 4.08 (9.3) |
9 5.0 (7.22) |
10 4.07 (6.02) |
11 17.01 (4.69) |
12 11.32 (10.67) |
13 2.01 (4.4) |
Table 1: Yields of bioconversion products and retention time in Gas Chromatography and Mass Spectrometry (GC- MS).
To clarify the time course of the microbial transformation of citronellol and citronellene, 100 mg of each compound was incubated with R. oryzae for 120 hrs. The broth solution (200 ml) was removed from the fermentation flask at intervals of 24 hours, which after extraction was analysed by Gas Chromatography (GC). It was observed that the citronellol progressed slower as compared to citronellene in product formation (Figures 3 and 4). Therefore, on the basis of these the conversion of citronellene was carried out for 48 hours, whereas citronellol was kept for 120 hrs. In both cases i.e. citronellol and citronellene one major metabolite compound Hydroxycitronellol and Dihydroxycitronellene respectively was detected. The formation of metabolites was determined based on the peak area of Gas Chromatography (GC). Compounds Hydroxycitronellol and Dihydroxycitronellene were identified as 7-hydroxy citronellol and 6,7-dihydroxycitronellene as major products respectively by Gas Chromatography (GC), GCMS, FT-IR, and high resolution 1H-NMR and 13C-NMR analyses.
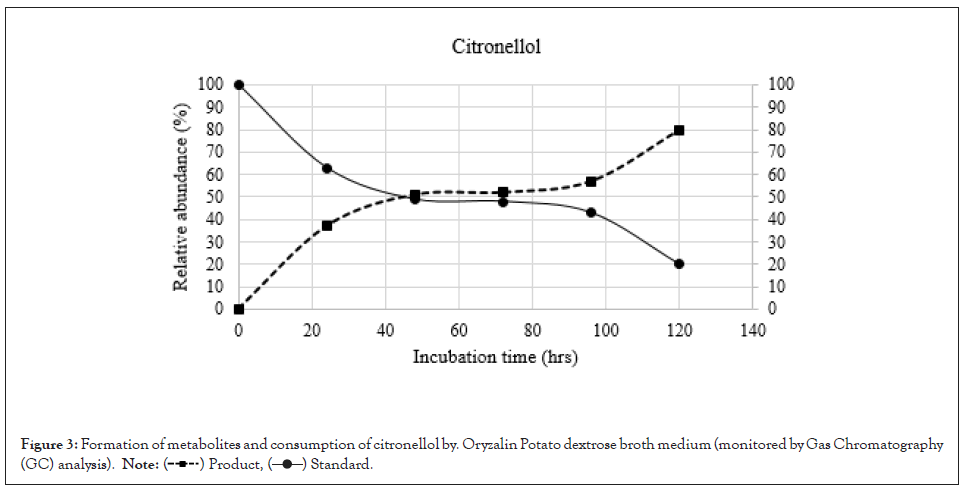
Figure 3: Formation of metabolites and consumption of citronellol by. Oryzalin Potato dextrose broth medium (monitored by Gas Chromatography (GC) analysis).
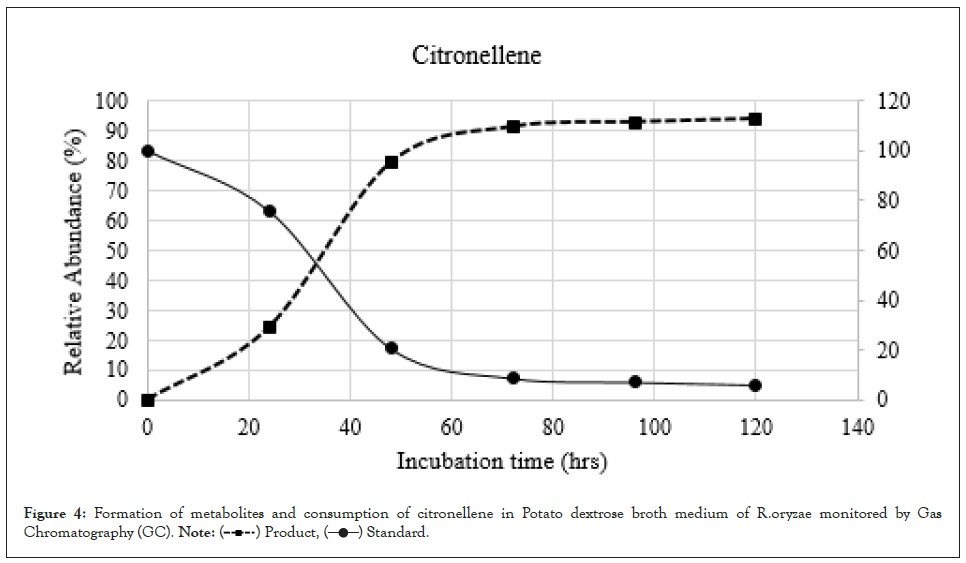
Figure 4: Formation of metabolites and consumption of citronellene in Potato dextrose broth medium of R.oryzae monitored by Gas Chromatography (GC). 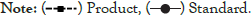
Products obtained from (S)-citronellol included 7-hydroxy citronellol, rose oxide, Rose glycol, isopulegol, 1,8-nonan-diol-8-methyl, artemisia triene, citronellyl-phenyl acetate (±) -Citronellene on similar treatment with R. oryzae was converted to 6,7-dihydroxy-citronellene, Isopulegone, terpinene-4-ol, camphene, camphor and α-pinene.
Spectral data of major products
7-hydroxycitronellol: Colourless oil; Yield 22.5%, [α] D -4.3 (c 1.5 MeOH), -4.19 (c 0.37 CHCl3), Chiral GC (rt) 28.05 min; GC/MS m/z 159 [M-Me]+ , 141 [159–H2O]+ , 123, 98, 83, 71, 59 (100%), 55, 53, 43. 159; IR νmax: 3360, 1470, 1381 Cm-1; 1H- NMR: δ 0.87 (d, J=6 Hz, 3H); 1.17 (s, 6H), 1.27-1.41 (m, 3H), 1.5-1.62 (m, 1H), 3.60- 3.68 (m, 2H); 13C- NMR: δ 19.7; 21.7, 29.3, 29.4, 29.5, 37.6, 39.8, 44.1, 61.0, 71.3. Spectral data of compound 3 fully agreed with an earlier report (Figures 5 and 6) [16].
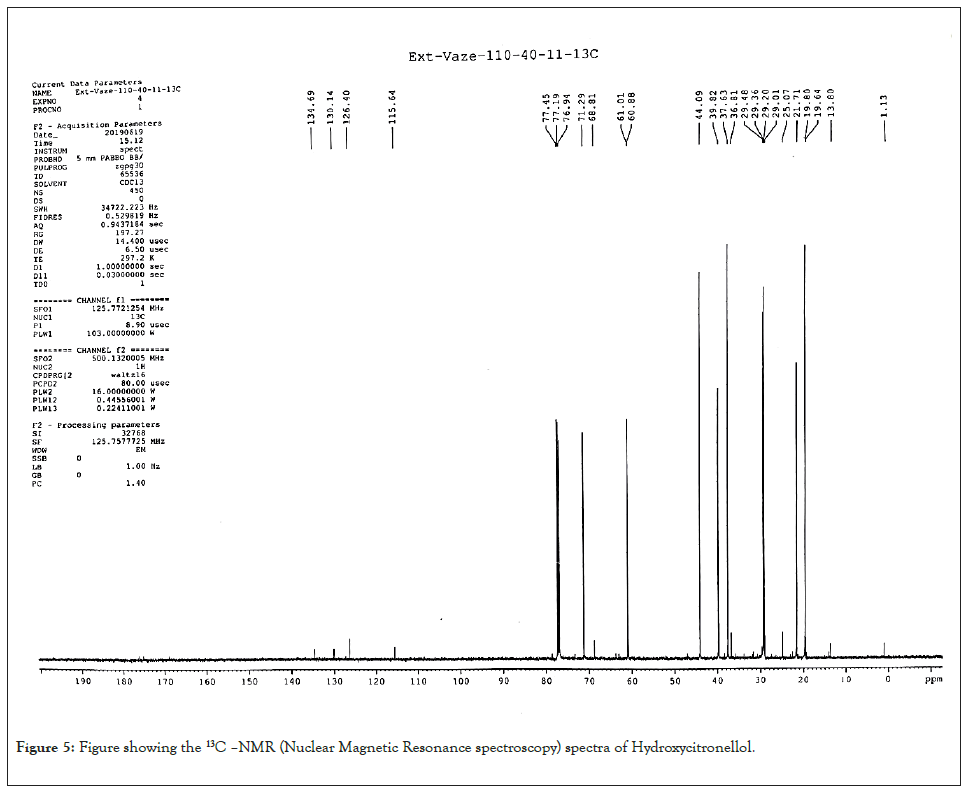
Figure 5: Figure showing the13C –NMR (Nuclear Magnetic Resonance spectroscopy) spectra of Hydroxycitronellol.
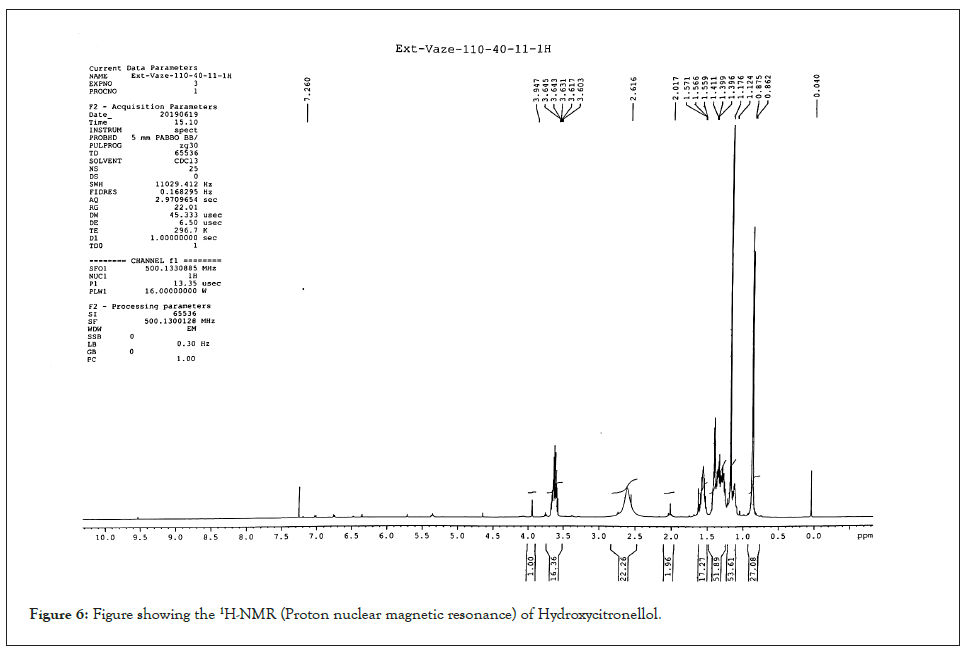
Figure 6: Figure showing the1H-NMR (Proton nuclear magnetic resonance) of Hydroxycitronellol.
6,7-dihydroxy-citronellene: Colourless oil; Yield 23%, [α] D -12.7 (c 0.384 MeOH); Chiral GC (rt) 24.09 and 24.32 min; GC/MS m/z 154 [M-H2O]+ , 139 [154–Me]+ , 121, 114, 96, 95, 81, 71, 59 (100%), 55, 53, 43; IR νmax: 3420, 1638, 1458, 1383, 1363 cm-1; 1H-NMR: δ 1.02 (3H, d, J=6.9 Hz) [1.01 (3H, d, J=6.9 Hz)]; 1.14 (3H, s), 1.20 (3H,s), 1.27-1.41 (3H, m), 1.5-1.72 (1H, m), 3.35 (1H, dd, J=10, 2 Hz); [3.32 (1H, dd, J=10, 1.5 Hz)]; 4.92 (1H, dd, J=10.3, 1.4 Hz), 4.98 (1H, dd, J=17, 1.2 Hz), 5.70 (1H, ddd, J=17, 10, 7.5 Hz) ; 13C-NMR: δ 20.7 (20.3); 23.3, 26.7, 29.4 (29.6), 33.6 (33.9), 37.9 (38.1), 73.4, 78.6 (79.1), 112.9 (113.2), 144.5 (144.7). Spectral data fully agreed with an earlier report [17] where Citronellelene was a metabolite in 3.1% yield, produced by larvae of common cutworm (Spodoptera litura) (Figures 7 and 8).
Figure 7: Figure showing the13C-NMR (Nuclear Magnetic Resonance spectroscopy) of the Dihydroxycitronellene.
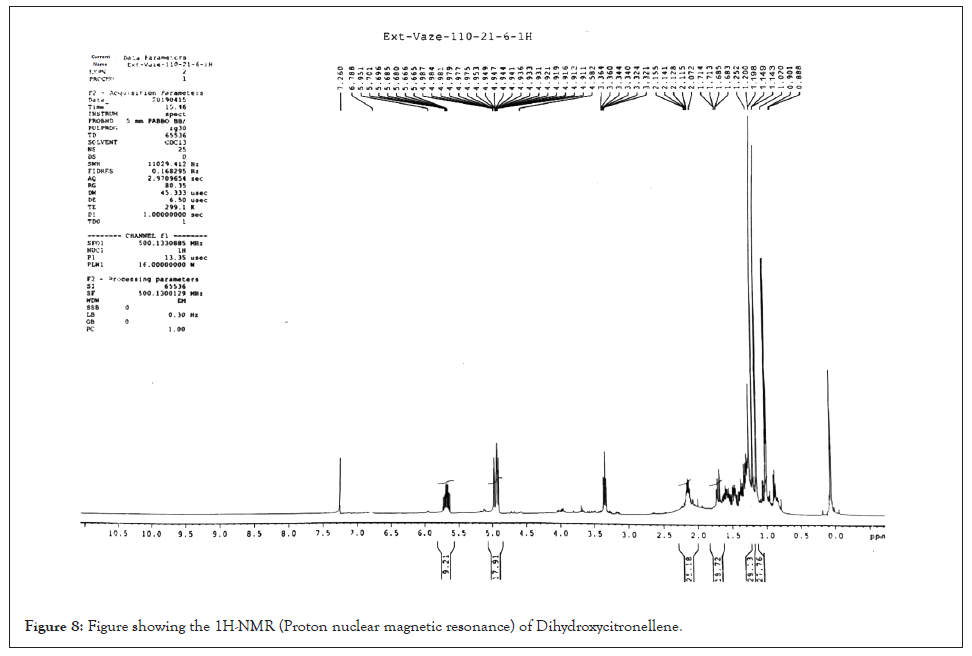
Figure 8: Figure showing the1H-NMR (Proton nuclear magnetic resonance) of Dihydroxycitronellene.
Gas chromatography/mass spectrometry data of minor products
Rose oxide- m/z 154, 139 (100%), 91, 83, 77, 69, 55, 41
Rose glycol-m/z 121 (M+ - 79), 99, 86, 71(100%), 55.41.
Isopulegol-m/z 154, 136, 121, 109, 95, 81, 69, 55, 41(100%).
1,8-Nonan-diol,8-methyl (8)-m/z 159, 141, 123, 98.1, 83.1, 70.1, 59.1(100%), 53, 41.
Isopulegone (9 -m/z 152, 137, 109, 95, 81 (100%), 67, 53, 41.
Terpinene-4-ol- m/z 154, 111, 93, 83, 71, 67, 55(100%), 43.
Camphene-m/z 136, 121, 106, 93(100%), 79, 67, 53, 43.
Camphor-m/z 153 (M++1), 137, 121, 108, 95(100%), 90, 81, 69, 55, 40
α -Pinene-m/z 136, 121, 105, 93(100%), 77, 67, 58, 53, 41
Artemisia triene-m/z 136, 121, 107, 93(100%), 79, 67, 55, 43.
Citronellyl- phenyl acetate-m/z 208 (M+-66), 192, 138.1, 123, 109, 95, 81 (100%), 69, 55, 41.
In this system, the substrates citronellol and citronellene were consumed in 120 hours and 48 hours respectively. Metabolites of compound citronellol increased gradually up to a level of 85% in 120 hrs. In contrast metabolites of compound citronellene increased gradually up to 90% in 48 hrs.
In the present study biotransformation of citronellene and citronellol by R. oryzae was studied, which produces various metabolites of different concentrations, which indicates that substrates are completely metabolized and the metabolites are formed mainly through enzymatic hydroxylation, hydration, epoxidation, esterification, degradation and rearrangement reactions through the enzymatic system of R. oryzae. Thus R. oryzae produces various enzymes for selective double-bond dihydroxylation, hydration, oxidative cyclization, and rearrangements. Citronellol thus gets converted to 7-hydroxy citronellol, and rose glycol through hydroxylation. Similarly, rose oxide and isopulegol are formed through oxidative cyclization. Artemisia-triene is formed through rearrangement. In citronellene, besides dihydroxylation, we observed skeletal rearrangements giving camphor, camphene, and α-pinene as products.
The IR spectrum of compound 3 showed the strong broad -OH band at 3360 cm-1 and 1H and 13C NMR spectra indicated the absence of the double bond. The δ values of peaks in 1H and 13C NMR were identical to the reported value of 7-hydroxy citronellol 3, which was obtained through microbial hydroxylation of tetrahydro-geraniol by Glomerella cingulata [18,19]. Other minor products identified through GC/EIMS analysis and matching the spectra from the NIST library, which included rose oxide, Rose glycol, isopulegol, artemisia triene, citronellyl- phenyl acetate. (±) -Citronellene on similar treatment with R. oryzae was converted to 6,7-dihydroxy-Citronellelene. The IR spectrum of compound Citronellelene showed the strong broad -OH band at 3460 cm-1 and 1H and 13C NMR spectra indicated the absence of one double bond. The δ values of peaks in 1H and 13C NMR were identical to the reported value of 6,7-dihydroxycitronellene which was the metabolite of cutworm when fed on citronellene [20-22]. Minor products included Isopulegone, terpinene-4-ol, camphene, camphor, and α -pinene.
R. oryzae has been a potential biocatalyst in bio transforming the monoterpenes into other natural metabolites having various applications. Moreover, it shows that some multistep or tedious reactions could be possible by using R. oryzae such asin the case of dihydroxy citronellene. Although efforts should be made to asses more different conditions with which specific product formation could be targeted and to study the enzymatic system of R. oryzae. Biocatalyst-based biotransformation has future potential as it is cheap, simple, eco-friendly, and less hazardous.
The authors would like to thank the Department of Science and Technology, New Delhi for the FIST grant and to Chemistry Department, Indian Institute of Technology, Bombay, and the for-NMR data.
No external funding was received.
The authors declare that they have no conflicts of interest.
Citation: Bhat SV, Mestry SJ, Gupta MO (2023) Biotechnological Transformation of Citronellene and Citronellol: By Fungus Rhizopus oryzae (ATCC 9363) Leading to Interesting Conversions through Oxidations and Rearrangements. Fungal Genom Biol. 13:207.
Received: 23-Jan-2023, Manuscript No. FGB-22-21131; Editor assigned: 26-Jan-2023, Pre QC No. FGB-22-21131 (PQ); Reviewed: 09-Feb-2023, QC No. FGB-22-21131; Revised: 16-Feb-2023, Manuscript No. FGB-22-21131 (R); Published: 23-Feb-2023 , DOI: 10.35248/2165-8056.23.13.207
Copyright: © 2023 Bhat SV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.