Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Short Communication - (2024)Volume 15, Issue 3
Erythropoietic Protoporphyria (EPP) is a rare, predominantly inherited photodermatosis in which exposure to visible violet light causes severe cutaneous pain. Deficiency of the ferrochelatase enzyme caused by FECH mutations leads to accumulation of Protoporphyrin IX (PPIX) in erythroid cells and endothelial cells. Light hitting the PPIX in dermal vessels causes free radical formation and vascular and dermal damage. Management to date has largely consisted of strict sunlight avoidance, pain relief for painful episodes, and liver monitoring due to the approximately 5% risk of acute liver failure [1]. Whilst bone marrow transplantation is curative, it is considered too risky to be considered in most patients, other than those with liver failure warranting liver transplantation. Antioxidants such as beta-carotene, vitamin E and oral cysteine, and bile-acid binding agents such as cholestyramine have been used with limited benefit [1]. Afamelanotide is a subcutaneous alpha melanocyte-stimulating hormone analogue, which received EMA approval in 2014 and FDA approval in 2019. It has become the standard of care for EPP in countries with access to it. By inducing eumelanogenesis without ultraviolet light exposure, afamelanotide reduces visible light penetration through the skin, thereby reducing the number and severity of phototoxic reactions [2]. It has also been shown to improve quality of life and psychosocial functioning [3]. Its antiinflammatory and antioxidant properties may also help ameliorate phototoxicity and hepatic damage [4]. Whilst afamelanotide is felt to be safe and significantly more effective than other treatments to date, with better performance than the clinical trials, it does not directly lower PPIX levels [5]. Hyperpigmentation of skin and mucosa, concerns regarding changes in naevi and the need for specialist training to administer the medications are other drawbacks.
Dersimelagon is an oral melanocortin 1 receptor agonist currently in phase 3 trials for EPP. It has a similar mechanism of action to afamelanotide, but a more convenient delivery [6,7].
Bitopertin is a novel, oral glycine uptake inhibitor which acts on the Glycine Transporter 1 (GlyT1) on erythroid cells. By limiting glycine uptake into cells, the haem pathway is downregulated, resulting in dose-dependent, statistically significant reductions in protoporphyrin IX. The BEACON trial is a phase 2, randomised open-label trial which enrolled 22 participants in Australia. The mean reduction in PPIX seen was approximately 40% for the 60 mg dose (p<0.001 vs. placebo) which translates into clinically meaningful improvements in the number and severity of phototoxic reactions and quality of life (Figure 1).
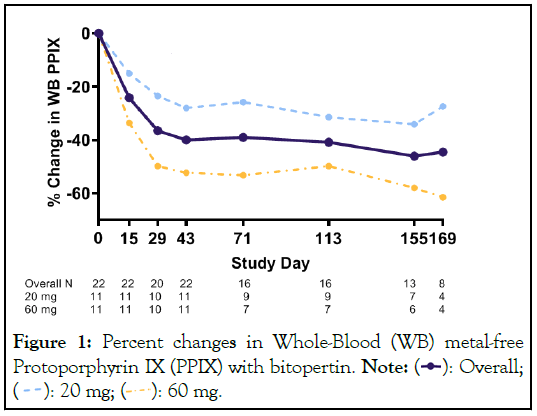
Figure 1: Percent changes in Whole-Blood (WB) metal-free
Protoporphyrin IX (PPIX) with bitopertin. Note: 
Patients on the BEACON trial reported being able to spend 3- fold longer in the sun before experiencing prodromal symptoms, and a 93% reduction in the number of phototoxic reactions. Nearly all participants (12/13) in BEACON reported that their EPP was much better or a little better by the end of the study. Improvements in PPIX levels were notably better in the 60 mg arm compared to the 20 mg arm. The drug was well tolerated, with no anaemia reported. Dizziness is the most common adverse event seen with bitopertin, along with headache and nausea. In the author’s experience, dizziness is temporary, lasting several days at the start of treatment before resolving spontaneously. There is some evidence that bitopertin can reduce cholestasis and liver fibrosis in the Fechm1PasEPP mouse, and this is another potential benefit which is being investigated in the current clinical trials [7].
In conclusion, the management of Erythropoietic Protoporphyria (EPP) has long relied on sunlight avoidance and symptomatic relief, with limited success. The advent of afamelanotide has marked a significant advancement, improving patients' quality of life by reducing phototoxic reactions. However, it does not directly address the underlying accumulation of Protoporphyrin IX (PPIX). The emergence of bitopertin, with its novel mechanism of action targeting glycine uptake, offers a promising solution. The BEACON trial results demonstrate clinically meaningful reductions in PPIX levels, leading to improved tolerance to sunlight exposure and a substantial decrease in phototoxic reactions. Additionally, the drug's favorable safety profile and potential to ameliorate liver complications suggest a multifaceted therapeutic approach. Bitopertin represents an exciting development in the management of EPP, offering hope for effective disease modification and improved outcomes for patients. Further research and clinical trials will be instrumental in fully elucidating its long-term efficacy and safety.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ross G (2024) Bitopertin for Erythropoietic Protoporphyria Lowers Protoporphyrin IX Levels and Improves Symptoms and Quality of Life. J Clin Exp Dermatol Res. 15:665.
Received: 01-Apr-2024, Manuscript No. JCEDR-24-30900; Editor assigned: 05-Apr-2024, Pre QC No. JCEDR-24-30900 (PQ); Reviewed: 19-Apr-2024, QC No. JCEDR-24-30900; Revised: 26-Apr-2024, Manuscript No. JCEDR-24-30900 (R); Published: 03-May-2024 , DOI: 10.35841/2329-9509.24.15.665
Copyright: © 2024 Ross G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited