Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2022)Volume 11, Issue 2
Duloxetine is a potent dual reuptake inhibitor of serotonin and norepinephrine receptors. Due to extensive first- pass metabolism, it has a major limitation of low and variable oral bioavailability. The present study aimed to develop a kinetically stable nanoemulsion using oil, surfactant, and co-surfactant for the better management of the major depressive disorder. The nanoemulsion was prepared by an aqueous titration method. The developed formulation was evaluated for pH, globule size, Transmission Electron Microscopy (TEM), iscosity, transmittance and further it was investigated for in vivo pharmacokinetic and bio-distribution study. According to the results of in vivo pharmacokinetic study in wistar rats, intranasal administration of duloxetine nanoemulsion in the brain had shorter Tmax compared with that of intravenous administration. The brain/blood ratios of duloxetine intranasal nanoemulsion, duloxetine intranasal solution, and duloxetine intravenous solution were 4.42 ± 0.09, 2.18 ± 0.14 and 0.19 ± 0.002 respectively. The higher drug targeting efficiency (381%) and direct transport percentage (73.7%) of duloxetine intranasal nanoemulsion as compared to other formulations indicate its better efficacy in the brain. In order to investigate the localization of duloxetine emulsion in brain confocal laser scanning microscopy was carried out using Rhodamine-123 (ROD-123) as a marker.
Duloxetine; Major depressive disorder; Intranasal nanoemulsion; Pharmacokinetic study; Sucrose preference test; Confocal study
Depression is a debilitating psychiatric disorder that affects more than 264 million populations worldwide. It’s the prevalence for longer duration manifests as serious health disorders which alter social well- being. The estimation for suicides each year has reached 800,000 and suicide is considered as one of the leading causes of death for children and adults in the age group of 15 to 29 years. Duloxetine (DLX) is one of the most commonly used medications for Major Depressive Disorder (MDD). It acts by inhibition of both serotonin and norepinephrine reuptake. According to the Biopharmaceutics Classification System (BCS), it is a class II drug indicating its limited solubility in water. Due to extensive first-pass metabolism, DLX shows very limited and variant bioavailability ranging from 40% to 80%. It also gets degraded by the action of acid present in the stomach as a result of which levels of DLX in the body reach below the desired therapeutic dose [1]. The Blood-Brain Barrier (BBB) is a fine structure situated in the endothelium of cerebral vasculature. It protects the brain from substances that have toxic potential and aids homeostasis. The fusion of capillaries is facilitated by tight junctions that have potent intercellular adherence [2]. Henceforth, the characteristic features of BBB result in restrictions for delivery and access through the Central Nervous System (CNS) [3]. Due to the limitations of the oral route, there is a dire need for innovative delivery systems which can efficiently deliver DLX. The intranasal route of administration offers a non-invasive delivery strategy to the brain. By utilizing the direct interconnection of the nasal mucosa and the CNS, the intranasal route bypasses the BBB which ultimately ameliorates the concentration of DLX in the CNS. The nasal mucosa has high vascularization in addition to the low enzyme inhabitation which further ensures higher amounts of DLX are delivered to the brain. DLX possesses all of the characteristics that make it a viable candidate for the development of intranasal nanoemulsion. A nanoemulsion is a promising formulation for intranasal administration of lipophilic drugs to the brain. Its bioacceptability, biodegradability, and fast uptake by the brain are all advantages of the lipid-based approach. These are isotropic, kinetically stable, transparent or translucent oil-aqueous phase dispersions stabilized by a surface active interfacial coating with droplet sizes ranging from 20 nm to 100 nm [4]. The present study evaluates the Intranasal Duloxetine Nanoemulsion (DLX-INE) relative to Intranasal Drug Suspension (DLX-INS) and Intravenous Drug Suspension (DLX- IVS). Complete pharmacodynamics and pharmacokinetic estimation for comparative evaluation was performed in the wistar rat model [5].
Materials
Duloxetine was gifted by Shodhana Laboratories Limited, Hyderabad, India. Capmul MCM was purchased from ABITEC Corporation, Janeswille, Wisconsin, United States. Labrafac, Labrafil, Labrasol, Transcutol HP and Capryol PGMC were obtained as a gift from Gattefosse, Saint Priest, Cedex, France. Groundnut Oil was purchased from Patanjali Ayurved Limited, Uttrakhand, India. Linseed oil was purchased from Lalit Hans Protein Private Limited, Rajasthan, India. Tween- 20 was purchased from Loba Chemie Private Limited, Mumbai, India. Peppermint oil was purchased from genuine chemicals, Mumbai, India. Di-sodium hydrogen phosphate anhydrous, Potassium dihydrogren orthophosphate (Anhydrous), High-Performance Liquid Chromatography (HPLC) grade acetonitrile and methanol HPLC grade were purchased from merck life sciences, Mumbai, India. Tween 80, Phosphoric Acid, Propylene Glycol, and PEG-400 were purchased from Thermo Fisher Scientific, Mumbai, India.
Animals
Wistar rats of either sex weighing 200-250 grams were utilized for pharmacodynamics, pharmacokinetic, brain targeting, and confocal microscopy investigations. The animals were provided with a generous supply of food and water ad libitum. Environmental conditions were maintained ambient. The temperature was adjusted at 23-30ᵒC and a 12-hour natural light-dark cycle. Ethical approval was approved by the Institutional Animal Ethical Committee (IAEC) of the University Institute of Pharmaceutical Sciences, Punjab University, India. The experimental protocols were conducted in accordance with the guidelines proposed by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India [6].
Analytical methodology
The duloxetine was analyzed by the HPLC method. HPLC was equipped synonym with gradient pump and variable wavelength of UV/Vis detector 2489, and waters e26395 software. Duloxetine was assayed by Reverse Phase High-Performance Liquid Chromatography (RP-HPLC). The mobile phase used was phosphate buffer/acetonitrile (60:40) with a 1 mL min-1 flow rate. Samples were filtered through a nylon filter (0.22 μm) before analysis and degassing was done by ultrasonic bath sonicator. The column was used of dimension 250 × 4.6 mm, 5 μm Oyster BDS Premium C18. The equipment was kept at room temperature. The analysis was performed by injecting 10 μL of sample and fixing detector wavelength to 230 nm [7].
Bio-analytical method and sample preparation
A bio-analytical method was used to estimate the concentration of duloxetine in the brain and blood using phosphate buffer and acetonitrile as the mobile phase. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) was carried out using a C18 column (250 × 4.6 mm, 5 μm Oyster BDS Premium). The samples were analysed at a flow rate of 1 mL min-1 and run time of 10 min the detection was performed at a fixed wavelength of 230 nm.
The liquid-liquid extraction technique was used to extract duloxetine from the plasma and brain samples. The plasma samples were prepared by collecting plasma (500 μl) and acetonitrile (500 μl) in an eppendroff and vortexing for 5 minutes to obtain a clear supernatant. For estimation of a drug in the brain, the whole brain samples were homogenised using a hand homogenizer. For each gram of brain sample, 3 ml of normal saline was added obtaining a ratio of 1:3. After homogenisation, the brain samples were cold-centrifuged for 10 mins at 10,000 rpm. The supernatant from both plasma and brain samples was extracted with the help of a syringe, filtered using a 0.22 μm filter, and transferred to HPLC via l s for analysis [8].
Screening of excipients
The solubility is an important parameter for preparing the nanoemulsion. The solubility of DLX was investigated in different oils (capmul mcm, groundnut oil, linseed oil, eucalyptus oil and peppermint oil) co-surfactants (propylene glycol, tween 20, labrafac, capryol, and transcutol) and surfactants (labrasol, labrafil, span 80, tween 80, and PEG 400). In a firmly closed eppendorf tube, the medication was introduced in excess to the excipients and well mixed using a vortex mixer. The dispersions were kept at 37ᵒC for 72 hours with agitation in a water bath shaker. They were centrifuged for 15 minutes at 3000 revolutions per minute (rpm), then filtered and diluted with an appropriate solvent (methanol) for analysis. UV spectroscopy with a maximum wavelength of 230 nm was used for the solubility study. Surfactant and co-surfactant were chosen based on oil miscibility and HLB value [9].
Construction of pseudoternary phase diagram
The pseudo ternary phase diagram was constructed to determine the exact proportion of each component needed in the formulation. The nanoemulsion was formulated using the aqueous phase titration method. The phase diagram was constructed using capmul mcm as the oil, labrasol as a surfactant and transcutol HP as co-surfactant, and distilled water as the aqueous phase. The surfactant and co-surfactant (Smix) were mixed at a ratio (2:1). Different combinations of oil and Smix were vortexed to obtain a clear and homogenous system, following gradual addition of water using micropipette under continuous stirring. The formulations were examined visually for cloudy or milky appearances or any phase separation. A pseudo ternary phase diagram of the oil, Smix, and water was developed using PCP triangular software with one axis reflecting the aqueous phase, the second axis reflecting oil, and the third axis reflecting a mixture of the surfactant and co- surfactant (Smix) at a fixed weight ratio [10].
Selection of nanoemulsion region from the pseudo ternary phase diagram
Based on the stability test and surfactant concentration, nanoemulsion was chosen from the pseudo ternary phase diagram. To form o/w nanoemulsions, the concentration of Smix was kept as low as possible whereas the concentration of water was kept at a maximum. The screened formulation was subjected to different physical stability studies which are heating-cooling cycle (4ᵒC ± 2ᵒC and 45ᵒC ± 2ᵒC, 6 cycles), freeze-thaw cycle (-20 ± 2ᵒC and+25 ± 2ᵒC, 6 cycles for 24 h), and centrifugation cycle (3500 rpm for 30 min). The formulation was observed for creaming, cracking, and phase separation [11].
Preparation of drug-loaded nanoemulsion
The drug-loaded nanoemulsion was formulated using the aqueous titration method using a vortex mixer (Remi equipment, Mumbai, India). A fixed quantity of DLX was made to dissolve in the oily phase, and then a predetermined amount of Smix was added while stirring continuously using a magnetic stirrer (Remi equipment, Mumbai, India). For a clear and homogeneous nanoemulsion, distilled water in a determined quantity was added drop wise to the mixture with continuous stirring [12].
Evaluation of duloxetine nanoemulsion
The prepared formulation was characterised for globule size, polydispersity index (Malvern panalytical) whereas the structural and morphological examination was performed using transmission electron microscopy (TEM, Hitachi, Tokyo, Japan). DLX loaded nanoemulsion was further evaluated by measurement of pH using pH meter (Cyberscan 510, E, Merck, USA) at room temperature and the viscosity was estimated by cup and bob viscometer (Anton paar, rheolab QC) at 37ᵒC. Percent transmittance (%T) of drug-loaded nanoemulsion was also calculated using UV spectroscopy at 230 nm [13].
Pharmacodynamic studies
A sucrose preference test was performed to investigate the antidepressant effect of the DLX-loaded nanoemulsion. Animals were randomly split into five groups. Each group consisted of five animals. Group A includes naïve rats. Group B includes control animals. To group C DLX loaded nanoemulsion was administered through the intranasal route. In group D duloxetine suspension was administrated intravenously and in group, E duloxetine loaded normal saline suspension was given through intranasal route. In groups, B, C, D, and E depression was induced by Chronic Unpredictable Mild Stress (CUMS) model as shown in Table 1 [14].
| Groups | Treatment | Route of administration | Dose (mg/kg) |
|---|---|---|---|
| A | Naïve | - | - |
| B | Control | - | - |
| C | DLX-INE | Intranasal | 0.54 |
| D | DLX-IVS | Intravenous | 0.54 |
| E | DLX-INS | Intranasal | 0.54 |
Table 1: The groups with their treatment schedule.
Sucrose preference test
As an anhedonia indicator, a sucrose preference test was performed. Animals were evaluated in this assignment to see how interested they are in a sucrose solution compared to ordinary water. Two bottles, one filled with 1% sucrose water (1 g sucrose in 100 mL water) and the other with ordinary water, were given to the animals throughout the training session for 24 hrs. The positions of the bottles were varied throughout the training session to avoid position preference. After 18 hours of water and food deprivation, a 6 hour test session was held. The sucrose preference was calculated using the equation below [15].
%Sucrose consumption=Sucrose solution (G)/Sucrose solution (G)+Water (G) × 100
In vivo study
For the study of pharmacokinetics and bio-distribution studies, wistar rats of either sex (200-250 g) were selected. The study was accomplished as per the principles of laboratory animal care and approved protocol by the Institutional Animal Ethical Committee of Punjab University, Chandigarh, India.
Pharmacokinetic and brain targeting study
For the pharmacokinetic studies, guidelines provided by the IAEC were followed. The animals were divided into five groups A, B, C, D, and E each containing 5 animals. Group A included normal rats termed as naïve. Group B to E all included depression-induced animals. Group B was termed as the Control. Group C (DLX-INE) was administered duloxetine loaded nanoemulsion through the intranasal route (volume 5 μL each nostrils) with a dose of 0.54 mg/kg. Group D (DLX- IVS) and E (DLX-INS) were given duloxetine suspension in normal saline via intranasal route and intravenous route (tail vein) of dose equivalent to 0.54 mg/kg/day respectively. The rats were anesthetized with the aid of diethyl ether and a blood sample (1 ml) was withdrawn Retino-orbitally. The animals were sacrificed at 1, 3, 6, 12, 24 h time intervals. The blood was transferred into microcentrifuge tubes that already contained EDTA serving as an anti-coagulant. The samples were centrifuged for 10 mins at 10,000 rpm. The brain sample was given washings with normal saline solution to eradicate the presence of unwanted tissue or fluid. The supernatant plasma and washed brain sample were stored in a deep freezer (-70ᵒC ± 10ᵒC) and consequently, utilized for further HPLC investigations [16]. The concentration of the drug in the brain and plasma sample was evaluated with aid of (PK Solver for Microsoft Excel Software). Several parameters like Area Under the Curve (AUC) were derived from the linear trapezoidal method, Maximum Plasma Concentration (Cmax), the time required to reach the Maximum Plasma Concentration (Tmax) were evaluated using the real plasma profile. Drug Targeting Efficiency (DTE%), which represents the drug's average time partitioning between the brain and the blood, and Direct Transport Percentage (DTP%) is the percentage of a drug that is transported directly to the brain via the olfactory and trigeminal neural pathways were determined. Further, percent absolute bioavailability (%F) was also calculated from the following equations [17].
DTE%=(AUC brain/AUC blood)in/(AUC brain/AUC blood)iv × 100
Where, (Bx=(Biv/Piv) × Pin)
Bx-The fraction of brain AUC through the BBB after intranasal administration
Bin-The AUC0-24 (brain) following IN administration.
Biv-The AUC0-24 (brain) following IV administration.
Piv-The AUC0-24 (blood) following IV administration.
Pin-The AUC0-24 (blood) following IN administration.
The percentage absolute bioavailability value (%F) of the Intranasal (IN) formulation was calculated as follows:
%F=AUCIN/AUCIV × DoseIV/DoseIN × 100
Bio-distribution studies by Confocal Laser Scanning Fluorescence Microscopy (CLSM)
Confocal Laser Scanning Fluorescence Microscopy (CLSM) was utilized to investigate the bio-distribution of DLX-NE using a fluorescent dye (i.e. ROD-123). Wistar Rats weighing 200-250 gm were utilized for the study. Induction of depression was done in all three groups in which formulation was administered C-E. Rhodamine-123 (ROD-123) is a fluorescent dye that has minimal ability to cross the BBB even when administered via the intravenous route. Initially, ROD-123 dye was dissolved in ethanol (10 mg/ml) than 0.175% (w/v) solution of ROD- 123 was blended in the oil phase of the emulsion. ROD-123 loaded nanoemulsion was administered intranasally to group C. To group D and E ROD-123 loaded solution was administered via intravenous and intranasal route, respectively. Animals were sacrificed using cervical dislocation at 30 mins, 60 mins, and 90 mins of administration to investigate localization of the nanoemulsion after which the brain was dissected. Consequently, the brain samples were washed with normal saline to eradicate the presence of unwanted tissue or fluid. The brain was cut with aid of a microtome into a thickness of 5 μm and fixed in a 10% (w/v) formaldehyde solution and observed under a fluorescent microscope. ROD-123 was observed as red fluorescent spots acting as evidence of drug-loaded into the emulsion [18].
Statistical analysis
Using the Turkey’s test, the differences between the groups were tested and the significance of data was evaluated by mean ± S.D at the level of 0.05. Three groups were compared using one-way ANOVA and the differences of p ≤ 0.05 were considered significant.
Screening of components
The oil is an important component of nanoemulsion formulation because it aids in the solubilization of lipophilic drugs. The possibility of drug precipitation exists if the drug is not freely soluble in the chosen oil. In the present study, the solubility of duloxetine was found to be highest in capmul MCM, which was 72.65 ± 0.11 mg ml-1. Therefore, capmul MCM was selected as the oil phase for the nanoemulsion.
The choice of surfactant is also crucial, as it must not only reduce interfacial tension but also be safe at the concentration employed. Ionic surfactants are thought to be more toxic than non-ionic surfactants. Non-ionic surfactants are known to be low in toxicity and chemically stable. The solubility and miscibility of the surfactants were used to screen surfactants. Labrasol was chosen as a result of these studies [19].
A surfactant alone rarely achieves transient negative interfacial tension and fluid interfacial film; therefore, a co-surfactant is required. The co-surfactant reduces bending stress and increases the flexibility of the interfacial film. Miscibility studies with the selected oil and surfactant were used to choose the co-surfactant [20]. It was found that duloxetine revealed maximum solubility in the transcutol HP. Also, transcutol HP has been reported to act as a permeation enhancer in nasal preparation. The comparison of different oils, surfactants, and co-surfactants was depicted using the bar graph given in Figure 1.
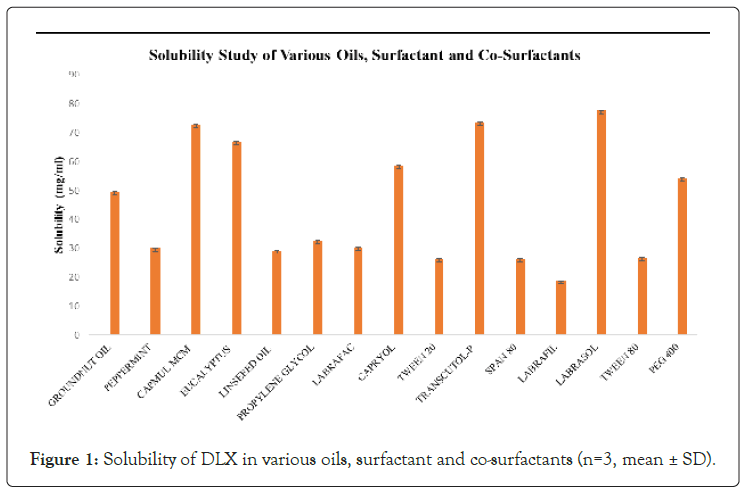
Figure 1: Solubility of DLX in various oils, surfactant and co-surfactants (n=3, mean ± SD).
Construction of pseudoternary phase diagram
Pseudoternary phase diagram was created using aqueous phase titrations to determine the surfactant to co-surfactant ratio capable of constructing the largest isotropic nanoemulsion region. Pseudoternary phase diagram was constructed using oil phase of capmul MCM (2:1), labrasol as surfactant, transcutol HP as co-surfactant, and aqueous phase as distilled water. The Smix ratio and its ability to solubilize the oily phase, as well as the system's decreasing free energy, were found to be directly related to the small or large area of emulsion. When the Smix ratio was increased the area of the nanoemulsion was slightly reduced, indicating that the surfactant was not contributing to the emulsification process. As a result, the emulsion region was chosen as 2:1 of the Smix as shown in Figure 2.
Figure 2: Pseudo ternary phase diagram containing the following components capmul MCM as oil, labrasol as surfactant, and transcutol HP as co-surfactant. Where dotted area shows oil in water nanoemulsion region.
Physical stability testing of nanoemulsion
A thermodynamic stable nanoemulsion system was prepared at a particular concentration of oil-water separation and Smix ratio. Creaming, cracking, and phase separation should be absent from the prepared formulation. The formulations with ratio system 2:1 was gone through different stress conditions, namely, heating-cooling cycle, freeze-thaw cycle, and centrifugation. Out of them, the formulation which passed all three stability tests was selected. The formulations which turned to be turbid were due to Oswald ripening [21].
Preparation of drug-loaded nanoemulsion
DLX was dissolved in the oil phase to prepare the Drug-loaded formulation. After that, the required amount of Smix was added, followed by a Drop-wise addition of distilled water until a clear and transparent liquid was achieved (Table 2).
| Batch no. | % of solvents | Heating and cooling cycle | Centrifugation cycle | Freeze thaw cycle | ||
|---|---|---|---|---|---|---|
| Oil | Water | Smix | ||||
| A1 | 5 (0.1 ml) | 5 (0.1 ml) | 90 (1.8 ml) | P | P | F |
| A2 | 5 (0.1 ml) | 15 (0.3 ml) | 80 (1.6 ml) | P | P | F |
| A3 | 5 (0.1 ml) | 25 (0.5 ml) | 70 (1.4 ml) | P | P | F |
| A4 | 5 (0.1 ml) | 35 (0.7 ml) | 60 (1.2 ml) | F | F | F |
| A5 | 5 (0.1 ml) | 45 (0.9 ml) | 50 (1.0 ml) | F | F | F |
| A6 | 5 (0.1 ml) | 55 (1.1 ml) | 40 (0.8 ml) | F | F | F |
| B1 | 10 (0.2 ml) | 10 (0.2 ml) | 80 (1.6 ml) | P | P | F |
| B2 | 10 (0.2 ml) | 20 (0.4 ml) | 70 (1.4 ml) | F | F | F |
| B3 | 10 (0.2 ml) | 30 (0.6 ml) | 60 (1.2 ml) | F | F | F |
| B4 | 10 (0.2 ml) | 40 (0.8 ml) | 50 (1.0 ml) | F | F | F |
| C1 | 15 (0.3 ml) | 5 (0.1 ml) | 80 (1.6 ml) | P | P | F |
| C2 | 15 (0.3 ml) | 15 (0.3 ml) | 70 (1.4 ml) | F | F | F |
| C3 | 15 (0.3 ml) | 25 (0.5 ml) | 60 (1.2 ml) | F | F | F |
| C4 | 20 (0.4 ml) | 35 (0.7 ml) | 50 (1.0 ml) | P | P | P |
| D1 | 20 (0.4 ml) | 75 (1.5 ml) | 0.5 (0.1 ml) | F | F | F |
| D2 | 20 (0.4 ml) | 65 (1.3 ml) | 15 (0.3 ml) | F | F | F |
| D3 | 20 (0.4 ml) | 55 (1.1 ml) | 25 (0.5 ml) | F | F | F |
| D4 | 20 (0.4 ml) | 45 (0.7 ml) | 35 (0.7 ml) | P | P | P |
| E1 | 30 (0.6 ml) | 10 (0.2 ml) | 60 (1.2 ml) | F | F | F |
| E2 | 30 (0.6 ml) | 20 (0.4 ml) | 50 (1.0 ml) | F | F | F |
| F1 | 40 (0.8 ml) | 10 (0.2 ml) | 50 (1.0 ml) | F | F | F |
| F2 | 30 (0.6 ml) | 20 (0.4 ml) | 40 (0.8 ml) | P | P | F |
| G1 | 50 (1.0 ml) | 40 (0.8 ml) | 10 (0.2 ml) | F | F | F |
| G2 | 50 (1.0 ml) | 30 (0.6 ml) | 20 (0.4 ml) | F | F | F |
| H1 | 60 (1.2 ml) | 30 (0.6 ml) | 10 (0.2 ml) | F | F | F |
| I1 | 70 (1.2 ml) | 25 (0.5 ml) | 5 (0.1 ml) | P | P | P |
| I2 | 70 (1.2 ml) | 20 (0.4 ml) | 10 (0.2 ml) | F | F | F |
| J1 | 80 (1.4 ml) | 15 (0.3 ml) | 5 (0.1 ml) | F | F | F |
Table 2: Physical stability testing of emulsion at Smix 2:1.
Evaluation of nanoemulsion
The nanoemulsion was transparent and monophasic, with a mean globule size of 54.64 nm ± 5.51 having a uniform PDI of 0.36 ± 0.154 (Figure 3). Because of the tightly packed surfactant films present at the oil-water interface, the higher the surfactant concentration, the stronger the stabilization. This suggests that the surfactant is more important than the co-surfactant in emulsifying the oily mixture [22]. Furthermore, the lower the PDI, the better the globule size distribution and formulation stability. Demonstrate morphology of formulated nanoemulsion was analysed via TEM studies (Figure 4). It was found that the optimized formulations had a spherical globule within a nanosize range of 48.455 ± 4.325 nm [23]. The globule size obtained in TEM analysis indicated the formulation of nanoemulsion. Also, the PDI obtained was supportive of the nanoemulsion minimising the chances of aggregation of globules (Table 3).
| Formulation components | Quantity % (w/v) |
|---|---|
| Capmul MCM | 20 |
| Labrasol | 33.3 |
| Transcutol HP | 16.7 |
| Water | 35 |
Table 3: Composition of nanoemulsion.
Figure 3: Shows the particle size of the optimized formulation.
Figure 4: TEM image of the optimized formulation. Note: Where 48.455 ± 4.325 nm is the size range of the spherical globule.
When it comes to optimizing intranasal formulations, viscosity is crucial because it is directly related to the time spent in the nasal mucosa. As a result, optimum viscosity of the formulation is desired to have a hassle-free administration, better penetration, and overcome mucociliary clearance. Using cup and bob viscometer, the viscosity of nanoemulsion came out to be 228 mPa-s (0.228 Pa-s) and shear rate and shear stress ranged between 0-100 S-1 and 0-20 Pa respectively (Figure 5). The linear correlation inferred that the nanoemulsion has the Newtonian type of flow [24].
Figure 5: Rheogram of optimized nanoemulsion.
The developed formulation's transmittance was found to be 98.15 ± 0.08%, indicating that the oil phase is completed dispersed in the aqueous phase and no aggregation was found owing to the stability of the nanoemulsion.
The pH of the nanoemulsion was found to be 5.87 ± 0.02, which lies in the normal pH range of the nasal cavity. It is one of the formulation considerations that would aid in the reduction of instillation-induced irritation [25].
Sucrose preference test
The sucrose preference test was used to determine anhedonia in this study. Anhedonia is a symptom of depression that is defined as the inability to derive pleasure from normally pleasurable experiences. It is one of the most common symptoms of depression. Rats suffering from depression were unable to tell the difference between the sweet sucrose solution and water, whereas healthy rats preferred the sucrose solution. Over the course of 24 hours, groups (C-E) treated with developed duloxetine nanoemulsion and duloxetine solution formulation showed improved sucrose preference when compared to the control group (B). Duloxetine-loaded formulations given via intranasal route showcased more effectiveness in contrast to Drug-loaded solution given via intravenous; the effects were quite similar to ones observed in naive group as shown in graph Figure 6. Values are expressed as mean ± SEM (Turkey’s test, one-way ANOVA, Graphpad Prism) where p ≤ 0.05 as compared to CUMS control group, p ≤ 0.05 as compared to CUMS DLX INE, CUMS DLX INS and CUMS DLX IVS [26-28].
Figure 6: Effect of DLX nanoemulsion on the percentage sucrose preference of the depression induced rats. Data represent the mean ± SD (n=3) P ≤ 0.05.
Pharmacokinetic studies and brain targeting
Mean plasma and brain concentrations versus time profile of duloxetine nanoemulsion formulation via IN and DLX solution via the IV and IN route was assessed to determine pharmacokinetic parameters. The concentration of duloxetine in the brain following IN administration of Nanoemulsion (NE) was higher at all time periods, but the remaining systems, DLX-INS and DLX-IVS, had lower concentrations (Figure 7a). The concentration of duloxetine in plasma after IV administration of DLX-IVS was shown to be significantly higher at all time periods in comparison to other DLX loaded systems administered intranasally, where it was found to be lower (Figure 7b). This found that intranasally delivered formulations had poorer drug distribution in systemic circulation than IV-administered formulations, indicating a preference for nose to brain delivery. explicate various pharmacokinetic parameters of duloxetine nanoemulsion administered via IN and duloxetine solution via IN and IV where the maximum brain concentration was calculated to be 9557.71 ± 153.07 ng/ml; Tmax 1 h, 5661.63 ± 300.63 ng/ml; Tmax 1 h, and 3538.17 ± 62.22 ng/ ml; Tmax 3 h, respectively (Table 4). The results clearly showed that the DLX concentration of the optimized nanoemulsion administered via IN was significantly higher (p ≤ 0.05) than that of the DLX suspension administered via IN and IV routes revealing targeted delivery of the formulation via olfactory route bypassing the BBB. The findings were in accordance with the outcomes of research illustrated in [28] who stated the usage of Labrasol and Transcutol which ultimately enhanced the drug penetration via nasal mucosa. The AUC0-24 of duloxetine in the brain after administration of DLX-INE (69007.48 ± 1201.55 ng h mL-1) was found to be significantly higher (p<0.05) when compared to DLX-INS (51636.78 ± 557.82 ng h mL-1) and DLX-IVS (45279.82 ± 175.61 ng h mL-1). DLX-INE showed nearly 1.3 folds higher AUC0-24 in the brain as compared to DLX-INS and 1.5 folds higher than DLX- IVS showing longer residence time of duloxetine nanoemulsion when administered via IN in the brain as shown by [29]. The brain/blood ratio of DLX for the optimized nanoemulsion IN and DLX solution IN and IV at 1 h were found to be 4.42 ± 0.09, 2.18 ± 0.14 and 0.19 ± 0.002, respectively demonstrating the superiority of an optimized DLX formulation for direct nose-to-brain delivery while avoiding the BBB whereas lower DLX-IVS was found because the drug reaches the brain via blood circulation crossing the BBB and minimizing its efficiency [12]. The elimination half-life (Ke) of DLX-INE given via the intranasal route was found to be significantly (p ≤ 0.05) higher in the brain at 0.18 h-1 that signifies ˜18% of the drug is excreted per hour which is 1.6 folds higher than DLX-INS and 4.5 folds higher than DLX-IVS [30]. DTE% and DTP% of the optimized nanoemulsion administered via IN were found to be 381% and 73.75% respectively due to target delivery of nanosized and lipophilic formulation. It was considerably higher in comparison to the DTE% and DTP% of DLX loaded solution given via IN which is 210.6% and 54.53% respectively. Duloxetine nanoemulsion, when administered intranasally, showed the presence of a greater amount of drug in the brain compared to the duloxetine solution (IN) which was also further evaluated by %F (Absolute bioavailability). DLX intranasal nanoemulsion was found to be 1.3 times bioavailable in the brain as compared to DLX intranasal solution thus the outcome of the result is in agreement with the study of who also predicted 1.5 higher bioavailability in brain of nanoemulsion [29]. This implies that the drug is transported from the nasal epithelium to the brain via the olfactory and trigeminal nerves, bypassing the BBB, which may not occur in systemic circulation [31].
| Formulation | Organ/tissue | Cmax (ng/ml) | Tmax (h) | T1/2 (h) | AUC0-24 (ng/ml.h) | Ke (h-1) |
|---|---|---|---|---|---|---|
| DLX-INE(in) | Brain | 9557.71 ± 153.07 | 1 | 8.51 ± 0.031 | 69007.48 ± 1201.55 | 0.18 |
| Blood | 2881.85 ± 86.93 | 3 | 28.66 ± 0.25 | 34525.62 ± 149.67 | 0.06 | |
| DLX-INS(in) | Brain | 5661.63 ± 300.63 | 1 | 22.62 ± 0.31 | 51636.78 ± 557.82 | 0.11 |
| Blood | 4193.85 ± 14.92 | 3 | 27.85 ± 0.54 | 46723.04 ± 348.41 | 0.07 | |
| DLX-IVS(iv) | Brain | 3538.17 ± 62.22 | 3 | 49.92 ± 3.7 | 45279.82 ± 175.61 | 0.04 |
| Blood | 9963.48 ± 11.87 | 1 | 15.96 ± 4.8 | 86320.16 ± 3064.3 | 0.12 |
Abbrevations: in: Intranasal; iv: Intravascular
Table 4: Pharmacokinetic parameters of DLX loaded nanoemulsion Intranasal (DLX-INE), DLX Intranasal Solution (DLX-INS), and DLX Intravenous Solution (DLX-IVS).
Figure 7a: Mean brain concentration of DLX versus time curve after DLX INE, DLX INS, and DLX IVS administration.
Figure 7b: Mean plasma concentration versus time curve after DLX INE, DLX INS, and DLX IVS administration. Data represents mean ± SD.
Bio-distribution studies
The samples of the brain with ROD-123 instilled was collected at three different time intervals at 30, 60, and 90 mins after IN and IV administration taking microscopic images to give results of the Bio- distribution study. Shows the concentration of ROD-123 in different regions of the brain after administration of ROD-123 emulsion IN ROD-123 solution IN and IV (Figure 8). Due to the existence of direct nose to brain transport bypassing the BBB, the tissue concentration in the form of the intensity of fluorescent dye of ROD-123 was higher in the brain with the ROD-123 nanoemulsion administered IN in comparison to the ROD-123 solution given IN and IV according to the findings of study [32]. Thus, the observed fluorescence is due to the ROD-123 embedded into the nanoemulsion which tends to increase its permeability.
Figure 8: Qualitative bio-distribution study of ROD-123 loaded (a) Intranasal Nanoemulsion (INE) (b), Intranasal Solution (INS), and (c) Intravenous solution (INV).
The present research work proposed a novel emulsion-based nanoformulation for the intranasal delivery of duloxetine. The study focused on formulating a nanosystem that is capable of Site- specific delivery to the brain via the intranasal route. The developed nanoemulsion is a promising delivery system for DLX. It exhibits significantly enhanced permeation through the nasal mucosal membrane. Experimental data supported the maximum in-vivo nasomucosal influx. Also, the concentration of drug in the brain was found to be higher proving the targeting efficiency of the prepared formulation through the intranasal route. The high concentration in the brain via intranasal nanoemulsion was supportive of the fact and the targeted delivery of DLX was achieved. Hence, intranasal administration of DLX avoids first-pass metabolism thereby enhancing the uptake of DLX to the brain. These research findings strengthen the suitability of intranasal nose to brain delivery systems for the treatment of a major depressive disorder.
The authors are grateful to Indian Council of Medical Research (ICMR), New Delhi for funding SRF fellowship to the first author (Ref. No. 45/57/2015/PHA-BMS). The authors are also thankful to faculty of Sophisticated Analytical Instrumentation Facility, Punjab University, Chandigarh for Transmission Electron Microscopy and Rheology Analysis.
The author confirms there are no conflicts of interest.
[Crossref] [Google scholar] 10
[Crossref] [Google scholar] 13
Citation: Singh G, Sidhu NK, Khanna G, Sarwal A, Kuhad A (2022) Brain Targeting of Duloxetine Nanoemulsion: In Vivo Pharmacokinetic and Bio-distribution Studies. J Develop Drugs. 11:168.
Received: 18-Feb-2022, Manuscript No. EOED-22-15923; Editor assigned: 22-Feb-2022, Pre QC No. EOED 22-15923 (PQ); Reviewed: 08-Mar-2022, QC No. EOED-22-15923; Revised: 14-Mar-2022, Manuscript No. EOED-22-15923 (R); Published: 21-Mar-2022 , DOI: 10.35248/2329-6631.22.11.168
Copyright: © 2022 Singh G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.