Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2023)Volume 14, Issue 4
Background: Brolucizumab is a new anti-Vascular Endothelial Growth Factor (anti-VEGF) in treatment of neovascular Age-related Macular Degeneration (nAMD) and Idiopathic Polypoidal Choroidal Vasculopathy (IPCV).
Materials and methods: A retrospective, consecutive, interventional studies from a tertiary eye hospital, where treatment naive and treatment switch patients were included. They underwent intravitreal injection Brolucizumab. Decision to reinject was made on presence of fluid on Spectral Domain Optical Coherence Tomography (SD OCT) or worsening of vision at follow-up. Outcome measures were change in Best Corrected Visual Acuity (BCVA), Central Subfield Thickness (CST) and changes in fluid (sub-retinal/intra-retinal/sub retinal pigment epithelium fluid) levels, Optical Coherence Tomography (OCT) biomarkers and safety analysis.
Results: A total of 59 eyes of 50 patients with a total of 132 intravitreal injections were included. There was a statistically significant improvement (p<0.05) in BCVA from baseline in treatment naive patients (mean BCVA at baseline 0.6 ± 0.41 and 0.37 ± 0.56). Mean baseline CST of all patients significant reduced from 582.92 ± 233.11 μm at baseline to 474.06 ± 252.89 μm at final treatment visit. Thirty-eight percentages of patients showed complete resolution of Sub- retinal Hyper-reflective Material (SHRM) after single injection. Interval between each subsequent injection increased from mean of 67 to 96 days in treatment switch and 47 to 151 days in treatment naive patients.
Conclusion: Brolucizumab promises reduced number of injections without any vision threatening complications.
Brolucizumab; Choroidal neovascular membrane; Sub-retinal fluid; Pigment epithelial detachment
Neovascular Age-related Macular Degeneration (nAMD) is a chronic progressive irreversible loss of vision due to macular degeneration affecting adults older than 65 years which is a leading cause among the elderly in developing countries like India [1]. The prevalence of nAMD in India ranges from 1.7%-3.3% [2]. It is estimated that AMD currently 196 million individuals worldwide are estimated to be affected by AMD which is expected to rise 288 million cases by 2040 [3].
Currently, intravitreal anti Vascular Endothelial Growth Factor (anti VEGF) injections are preferred treatment for Choroidal Neovascularization Membranes (CNVM) secondary to nAMD. The challenge of treating nAMD is multidimensional including burden of multiple intravitreal injections. An emotional burden where the patient is stressed at undergoing multiple procedures; a logistic burden, where there maybe no one to accompany the patient for clinic visits and the financial burden, as most of these patients are retired and may not have a steady source of income or the caregiver may need to miss a day of work to come along with the elderly [4].
Food and Drug Administration (FDA) approved Ranibizumab in 2004, and aflibercept in 2011 the United States, and their efficacy and safety has been established for treatment of nAMD [5].
With the recent approval of brolucizumab, the neovascular AMD armamentarium has expanded. There is always a need for a newer molecule that is longer lasting and more effective, increasing the treatment interval and decreasing the morbidity and treatment burden, and in our experience, Brolucizumab promises to be that molecule. It is a rabbit derived humanized, single-chain variable fragment (scFv) antibody with a molecular mass of ~ 26 kDa that inhibits all isoforms of VEGF-A [6].
We share our real-world data describing the efficacy and safety profile Brolucizumab in the treatment of nAMD and Idiopathic Polypoidal Choroidal Vasculopathy (IPCV).
This was a retrospective, consecutive, interventional, nonrandomized study from Rajan eye care Hospital, Chennai, a tertiary eye care institute in South India from January 2021 to June 2022. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at Rajan eye care. Written informed consent for treatment and data collection was obtained from each patient.
The data of all consecutive patients who were treated with intravitreal injections of Brolucizumab was accessed from the electronic medical records. The inclusion criteria was the presence of an active CNVM complex indicated by fluid (sub-retinal, intra- retinal and sub-retinal pigment epithelium fluid) on Spectral Domain Optical Coherence Tomography (SD OCT), and/or showed leakage or hyperfluorescence of indeterminate origin on fundus fluorescein angiography and/or polyps on indocyanine green angiography. The patients were both treatment naive and those who had been previously treated with other anti-VEGF agents with negligible results/recalcitrant CNVMs (persistent fluid of 100 microns or more) or worsening vision. The risks and benefits of all anti-VEGF agents were explained, and they were free to choose to receive Intravitreal (IV) Brolucizumab.
The exclusion criteria included eyes with media opacities that precluded the observation of the ocular fundus, history of any retinal surgery, coexisting diabetic retinopathy or glaucoma, any history of systemic autoimmune disease, any history of preexisting uveitis/vasculitis and CNVM due to causes other than nAMD.
At baseline and all subsequent follow-up visits, details of the clinical examination including Best Corrected Visual Acuity (BCVA) assessment using the Snellen’s visual acuity chart, Intraocular Pressure (IOP) measurement, anterior segment evaluation using slit-lamp biomicroscopy and fundus examination with indirect ophthalmoscopy and SD-OCT (Cirrus 5000, Zeiss) were recorded. All patients had received intravitreal injection Brolucizumab (6 mg in 0.05 mL) in the operating room under sterile precautions. Post operatively, they were seen in the clinic within the first 48-72 hours and then 6 weeks later. Any history regarding the occurrence of any ocular or systemic adverse event was noted. The decision to reinject was made on the presence of fluid on SD OCT or worsening of vision at the end of 6 weeks, based on Pro Re Nata (PRN) regimen. Outcome measures were any change in BCVA, in Central Subfield Thickness (CST) from the baseline and changes in the fluid (sub-retinal/intra- retinal/sub Retinal Pigment Epithelium (RPE) fluid) levels and OCT biomarkers along with safety analysis. All imaging analysis was performed by two independent graders such as Standard Deviation (SD) and Absolute Mean (AM).
Statistical analysis
This was a real-world study; the categorical variables were represented as absolute mean and standard deviation. The comparisons of the categorical variables between the groups of different indications were performed using student paired t-test. A p-value<0.05 was statistically significant. Last observed carry forward values from final visits were considered to make up for possible lost follow-ups. BCVA was measured using Snellen’s chart and converted into logarithm of the Minimum Angle of Resolution (log MAR) values in decimal unit [7,8].
A total of 59 eyes of 50 patients were included in the study. Among our study population, 21(37.5%) were treatment naïve and 38(62.5%) were previously treated with anti-VEGF injections (mean 2.3 injections per eye). Type 1 CNVM was seen in 43 eyes (71.43%), Type 2 CNVM in 2(3.57%), Type 3 CNVM in 3(5.36%) eyes. Eleven eyes (19.64%) had Polypoidal Choroidal Vasculopathy (PCV). Of all the patients recruited 66.07% had a history of cardiovascular disease and 19(33.93%) had type 2 diabetes mellitus. 10 patients had unilateral pathology and remaining 36 patients had bilateral pathology. Table 1 shows the demographic characteristics of the study population (Table 1).
| Parameter | N(%) |
|---|---|
| No. of patients | 46 |
| No. of eyes | 59 |
| Age | 74.06 ± 5.6 years |
| Males | 24 |
| Females | 22 |
| Type of CNVM (eyes) | |
| Type 1 | 43(71.43%) |
| Type 2 | 2(3.57%) |
| Type 3 | 3(5.36%) |
| PCV | 11(19.64%) |
| History of CVD and T2DM | |
| Yes | 37(66.07%) |
| No | 19(33.93%) |
| Total Number of Injections | 132 Injections |
| Previous treatment status (eyes) | |
| Previously treated | 38(62.5%) |
| Treatment naive | 21(37.5%) |
| BCVA | 0.60 + 0.4 LogMar (6/23) |
| Patients with unilateral injection | 10(21.74%) |
| Patients with bilateral injection | 36(78.26%) |
Note: CNVM: Choroidal Neovascular Membrane; PCV: Polypoidal Choroidal Vasculopathy; CVD: Cardiovascular Disease; T2DM: Type 2 Diabetes Mellitus.
Table 1: Demographic characteristics of the study population.
Change in best corrected visual acuity
There was a statistically significant improvement (p<0.05) in visual acuity from the baseline in treatment naive patients (mean BCVA at baseline 0.6 ± 0.41 and 0.37 ± 0.56). The visual acuity in previously treated patients was 0.6 ± 0.43 at baseline and 0.7 ± 0.24 at the final visit. In these subgroups of patients an improvement in visual acuity was seen in second visit after which there was a worsening which could be explained by scarring of CNVM with or without RPE atrophy (Figure 1).
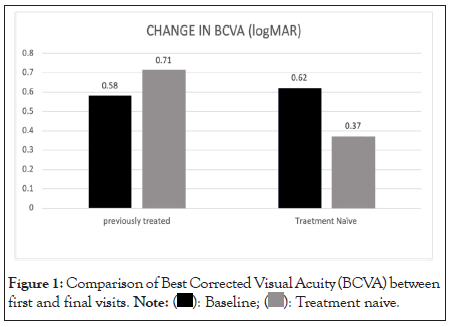
Figure 1: Comparison of Best Corrected Visual Acuity (BCVA) between
first and final visits. 
Injection interval
In the subgroup analysis, the injection interval between first and second and second and third injections was 47.37 and 45. 86 days in the treatment naive group, and 67.86 and 73.43 days in the previously treated group respectively. All the subjects in our study were treated Pro Re Nata (PRN) protocol. In patients who had received five injections, the mean injection interval was successfully titrated up to 97.8 and 115.9 days between third and fourth injection and fourth and fifth injection respectively (Table 2 and Figure 2).
| Categories | Interval dose 1 and 2 | Interval dose 2 and 3 | Interval dose 3 and 4 | Interval dose 4 and 5 |
|---|---|---|---|---|
| Previously treated | 67.86 | 73.43 | 84.86 | 96.81 |
| Treatment Naive | 47.37 | 45.86 | 123.87 | 151.79 |
Table 2: Table showing the mean interval (days) between two subsequent doses in previously treated and treatment naive patients.
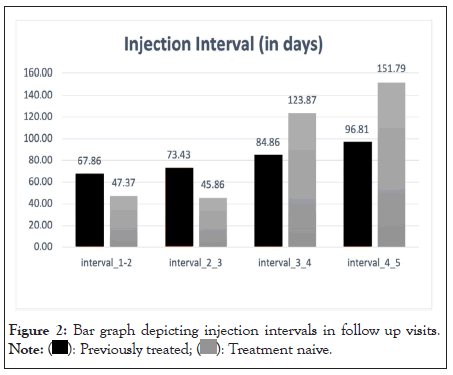
Figure 2: Bar graph depicting injection intervals in follow up visits. 
Change in central subfield thickness
The mean baseline CST of all patients at baseline was 582.92 ± 233.11 μm which reduced to 474.06 ± 252.89 μm at the final treatment visit. This difference was statistically significant (p<0.05). Subgroup analysis revealed a statistically significant reduction in CST in the treatment naïve group from an initial mean 550.79 ± 214.09 μm to 377.17 ± 245.73 μm (p<0.0001). The CST of previously treated group, also showed a statistically significant change from a baseline of 603.10 ± 243.6 μm to 501.41 ± 249.1 μm (p=0.05) (Figure 3).
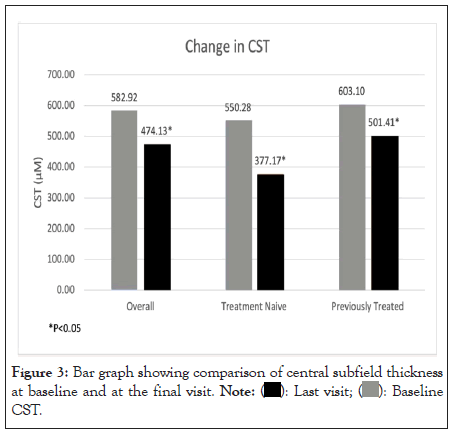
Figure 3: Bar graph showing comparison of central subfield thickness
at baseline and at the final visit. 
Changes in OCT biomarkers
48.8% of patients showed complete resolution of sub-retinal fluid after single IVI brolucizumab. 18.42% of patient and 37.5% of patients showed complete resolution of intra-retinal fluid and SHRM post single injections. Table 3 shows the response of various biomarkers after a single IVI brolucizumab (Table 3).
| OCT biomarkers | At baseline | Post 1st injection |
|---|---|---|
| Sub-retinal fluid | 43/59(72.9%) | Resolution 21/43(48.83%) |
| Reduction 7/43(16.27%) | ||
| Intra-retinal fluid | 38/59(64.4%) | Resolution 7/38(18.42%) |
| Reduction 15/38(39.47%) | ||
| Pigment epithelial detachment | 41/59(72.9%) | Resolution 5/41(12.19%) |
| Reduction 18/41(43.9%) | ||
| Sub-retinal hyper-reflective material | 32/59(54.23%) | Resolution 12/32(37.5%) |
| Reduction 10/32(31.25%) | ||
| Shallow irregular RPE elevation | 26/59(44.06%) | Resolution 10/26(38.46) |
| Reduction 3/26(11.53%) |
Note: OCT: Optical Coherence Tomography; RPE: Retinal Pigment Epithelium.
Table 3: Changes in Optical Coherence Tomography (OCT) biomarkers at baseline and after 1st injection Pagenax.
Safety
In our case series, we encountered 3 patients with serious adverse events. We had a 61 year old lady, k/c/o Idiopathic Polypoidal Choroidal Vasculopathy (IPCV) who was switched to brolucizumab after a poor response to the latest VEGF. After her first dose of brolucizumab, she developed grade 4 vitritis. A working diagnosis of infectious endophthalmitis was made and she underwent a 23 gauge emergency pars plana vitrectomy with intravitreal vancomycin and ceftazidime. Postoperatively, the vitritis resolved and so did the sub-retinal fluid. Patient remained fluid free for 7 months post injection. She went on to get another 3 injections of brolucizumab with prolonged intervals in between, with no further episodes of inflammation.
The second patient was an 82-year-old man, underwent consecutive injection in both eyes for treatment naive CNVM. He was doing well, till 80 days later, he developed drop in vision with vitritis, retinal sheathing, cotton wool spots and a RPE rip, juxtafoveal in the right eye. The uvea blood work was done and negative. He was started on an intensive dose of oral steroids (1 mg/kg/body weight) and topical steroids and topical cycloplegics. He responded well and vitritis resolved. The third patient developed a full thickness macular hole after 6 weeks of the third injection of brolucizumab.
The study has demonstrated a significant improvement in the anatomical outcomes with a statistically significant improvement in central sub-foveal thickness with overall resolution in Sub- retinal Fluid (SRF), Intra-retinal Fluid (IRF), sub-PRE fluid, SHRM, and Shallow Irregular Retinal pigment Epithelium (SIRE) consistently in both treatment naive and previously treated group this shows a favourable response to anatomical change of the disease. The overall vision was maintained without a significant reduction during the treatment with Brolucizumab.
Since the inception of the anti-VEGF in the treatment age related macular degeneration the quest for more durable agent with superior drying of retinal fluid has started, Brolucizumab was approved the treatment of nAMD. The Phase 3 HAWK and HARRIER trial has shown the brolucizumab increase injection interval can be increased q12w soon after loading phase with significant reduction in IRF/SRF and sub-RPE fluids. The results of the study was consistent with the phase 3 study with significant improvement in anatomical outcome and with an extension of injection interval of more the 120 days.
Compared to the other real-world studies which has shown a significant improvement in Visual Acuity (VA) our study did not show a significant improvement in change in VA from the baseline, the result was consistent in both treatment naive and previously treated group [9,10]. Studies have shown a good baseline likely to maintain good VA outcomes in long term, in our study the patient presented with wide range of baseline VA starting from counting fingers to a vision of 6/6 this variation may be a reason for VA outcomes [11]. Also there are reports of ceiling effects with anti-VGEF may also account for varying VA results [12].
The study has shown a significant reduction in SD-OCT markers. There was a significant reduction in CST9, also a there was good resolution of IRF, SRF, Pigment Epithelial Detachment (PED), SHRM and SIRE [13]. The study result was in consistent with the previously published studies were there was good resolution of fluid [14,15].
Numerous studies have proved the efficacy of Brolucizumab, but there have been concerns of safety. The choice of treating any AMD patient with Brolucizumab is an understanding of the risk- benefit ratio. Studies have shown similar risk of moderate vision loss between aflibercept and brolucizumab, despite published cases of retinal vasculitis and retinal vein occlusions [16]. The only identified risk factors for intraocular inflammations were the female sex, Japanese ethnicity and known cases of autoimmune disease and hence a judicious choice of appropriate patients.
Despite many Indian studies having reported nil cases of intraocular inflammation, we reported 3 cases of adverse events. They however, resolved with treatment and vision was stabilized. Most of the intraocular inflammations reported have occurred at an average of 26 days; however our cases had a variable interval from 40 days to 80 days post injection. It could be just one injection or repeat injections that cause the cascade of inflammation. In most of the reported cases, the inflammation resolved with oral, topical, and intravitreal steroids. However, the role of prophylaxis does not justify the number needed to treat. Other potential serious adverse events associated with brolucizumab include hypersensitivity, endophthalmitis and retinal detachments, increased intraocular pressure, and systemic arterial thromboembolic events were noted in HAWK and HARRIER studies.
Brolucizumab (dosed every 8 or 12 weeks) was found to be non- inferior to aflibercept (dosed every 8 weeks) in the phase 3 clinical trials, HAWK and HARRIER. When compared to eyes treated with aflibercept eyes treated with brolucizumab had greater reductions in retinal thickness.
Brolucizumab has proven its efficacy in treating the CNVM complex in nAMD and IPCV. It helps reduce the subretinal, intraretinal and sub RPE fluid along with reduction in SHRM and SIRE. Our study did not show any vision threatening complications. The risk of RPE rip is not greater than the other anti VEGF injections. However, a judicious choice of patients and appropriate counselling is advocated prior to the injections.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Dabir S, Mohankumar A, Khatri M, Rajan M (2023) Brolucizumab in Age-Related Macular Neovascularization (Brain Study): Efficacy, Optical Coherence Tomography Biomarkers and Safety Profile over 18 Months. J Clin Exp Ophthalmol. 14:954.
Received: 29-Jun-2023, Manuscript No. JCEO-23-22286; Editor assigned: 03-Jul-2023, Pre QC No. JCEO-23-22286 (PQ); Reviewed: 17-Jul-2023, QC No. JCEO-23-22286; Revised: 24-Jul-2023, Manuscript No. JCEO-23-22286 (R); Published: 31-Jul-2023 , DOI: 10.35248/2155-9570.23.14.954
Copyright: © 2023 Dabir S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.