Medical & Surgical Urology
Open Access
ISSN: 2168-9857
ISSN: 2168-9857
Research Article - (2021)Volume 10, Issue 9
Purpose: To study the association of prostatic volume, prostatomegaly grade and IPSS (International Prostate Symptom Score) with vitamin D deficiency if any.
Materials and Methods: The study was conducted on 150 patients, 50 to 70 years of age. Patients presenting with LUTS (Lower Urinary Tract Symptoms) were randomly selected for the study. A diagnostic evaluation for LUTS was done in all the patients based on IPSS & clinical examination. Prostate volume was calculated using Ultrasonography (USG) and serum Prostate Specific Antigen (PSA) cut off of <4 ng/ml was considered for inclusion. Successively all the patients were tested for serum 25-hydroxyvitamin D 25(OH) D level. Patients diagnosed with vitamin D deficiency were classified as “study population” and those with normal vitamin D level were classified as "controls”.
Results: The median serum level of 25(OH) D in the participants was 18.95ng/ml. Vitamin D deficiency was detected in 70% of the participants. The study population had a significantly greater mean prostate volume of 41.32 ± 23.29 ml than 23.42 ± 8.62 in controls (P <0.001). The study population also had significantly greater prostatomegaly grade on DRE and IPSS than controls (P=0.001).
Conclusion: Vitamin D deficiency is associated with higher prostate volume and IPSS score in patients with BPE. If further studies confirm this association, treatment of vitamin D deficiency may help to prevent or delay symptoms of BPE.
Vitamin D deficiency; Benign prostatic hyperplasia; Prostate volume; IPSS
Key message: Vitamin D deficiency is quite prevalent in ageing men. It may have a significant role in the etiopathogenesis of benign prostatic enlargement and its symptomatology. Treatment of Vitamin D deficiency may help prevent or counteract the symptoms of benign prostatic enlargement.
Benign Prostatic Enlargement (BPE) is one of the most prevalent ageing-related derangements in men and has a significant impact on quality of life [1]. Prevalence of BPE ranges from 40-50% at the age of 50, 80% at the age of 70 and about 90% by the ninth decade [2]. It is entrenched that normal, as well as malignant prostate cells, express vitamin D receptors, [3] which are responsible for regulating cell growth and replication [4,5]. Vitamin D3 and a few of its analogues can be used to modulate the growth and differentiation of prostatic cells [6]. Consequently vitamin D deficiency could potentially be a modifiable risk factor for the prevention of BPE in addition to other risk factors like obesity, diabetes mellitus, diet and lifestyle [7]. We aimed to study the association of prostatic volume, prostatomegaly grade and IPSS (International Prostate Symptom Score) with vitamin D deficiency if any.
After obtaining approval from the Institutional Ethics Committee, an observational case-control study was conducted at our hospital from May 2017 to December 2018. One fifty men aged between 50 to 75 years, presenting with BPE and LUTS with a negative urine dip for urinary tract infection and PSA<4 ng/ml were enrolled in the study. All the patients had voluntarily consented for their inclusion in the study. Patients with a history of neurogenic bladder, stricture urethra, carcinoma of bladder or prostate, vesical calculi and serum PSA level > 4 ng/ml were excluded from the study. Patients with PSA of more than 4 ng/ml and a family history of prostatic malignancy were referred to a cancer clinic for further evaluation.
Clinical variables
The prostate gland was assessed by digital rectal examination for size grading and the Siemens Acuson X-300 colour Doppler machine was used for prostate volume measurement by a fixed assigned clinician. Participants were instructed to fill the International Prostate Symptom Score (IPSS) questionnaire [8] which includes 7 questions, having each response to be scored from 0 to 5, giving a maximum score of 35 points. The response was graded as 0-7 being mildly symptomatic, 8-19 being moderately symptomatic, 20- 35 being severely symptomatic. Prostatomegaly grading was done as per classification described by Romero, et al. [9]. Working diagnosis of BPE was made depending on the presence of LUTS, prostate volume greater than 20 ml and serum PSA less than 4ng/ml.
Blood Sampling and Laboratory Testing: For submitting blood samples patients were instructed to observe overnight fast and abstain from smoking for at least 12 hours. Centrifuged samples were immediately stored at−80°C before assay. The circulating form of vitamin D (25OHD) was assayed by liquid chromatography with mass spectrometry detection (LC-MS/MS). Eurolyser CUBE – S kit was utilized for measuring serum PSA level. Vitamin D levels were classified as vitamin D deficiency (level <20 ng/ml), vitamin D insufficiency (level - 20-30 ng/ml) and normal level (level >30 ng/ml).
Statistical analyses
Result were expressed as medians (interquartile ranges) or (mean ± standard deviation) for the quantitative variables and percentages for qualitative variables. Qualitative variables were compared between groups using the Chi-square test. Mann-Whitney U test was done to compare the difference in the distribution of DRE grades and IPSS. Quantitative variables were compared using the T-test. Participants were divided into 2 groups, based on serum 25(OH) D levels, namely, study population (BPE with vitamin D deficiency, level <20 ng/ml) and control group (BPE with vitamin D insufficiency, level 20-30 ng/ml or normal level >30 ng/ml).
Associations of vitamin D deficiency with prostate volume, grade and IPSS were assessed using linear regression models in multivariate adjustment, for possible confounders. The results were expressed as odds ratios (ORs) with the corresponding 95% CI. Statistical significance was defined as P value <0.05. Statistical analysis was performed using SPSS for Windows (version 24.0. Armonk, NY: IBM Corp).
150 participants were included in the study. The median serum level of 25(OH) D in our participants was 18.95 ng/ml. Vitamin D deficiency was detected in 70% of participants, which were classified as study population & the rest 30% were classified as controls. Compared to the controls the median serum level of 25(OH) D in the study population was significantly lower (12.75 ng/ml [interquartile range, 11.22 - 15.76] vs 14.84 ng/ml [interquartile range, 12.05-21.59].
Prostate Volume: As shown in table 1, the mean prostate volume in the study population was 41.32 ml, which was 23.42 ml in controls. The difference between the two was statistically significant. The prostate volume was greater than 20 ml in 65.1% of cases with vitamin D deficiency versus 34.2% of control subjects (P <.003). There was a significant negative correlation between 25(OH) D level and prostate volume which suggests lower the 25(OH) D levels higher the prostate volume (r = −0.328, P < .001).
| Parameters | Study population= 105 | Controls N1=45 | P value |
|---|---|---|---|
| Prostate volume, cc (mean value ± SD) |
41.32 ± 23.29 | 23.42 ± 8.62 | <0.001* |
| Prostatomegaly grade | Grade I= 41.9 % Grade II= 35.2 % Grade III= 22.9 % |
Grade I= 62.67 % Grade II= 33.33 % |
0.001* |
| IPSS | Mod/Severe= 78.6 % Mild= 21.4 % |
Mod/Severe= 21.4 % Mild= 55.3 % |
0.001 |
| Vitamin -D Level (ng/ml) median, (interquartile range) |
12.75 (11.22 - 15.76) | 14.84 [12.05-21.59]. |
Values are represented as mean value ± SD, percentages and numbers.
*significant
Table 1: Comparison for prostate volume, prostatomegaly grade, IPSS and Vit-D level between study population and controls.
Prostatomegaly grade: When the grade of prostate size was assessed by digital rectal examination (DRE) in the study population its distribution was observed as 41.9%, 35.2% and 22.9% for grade I, II and III respectively. While in the controls its zdistribution was 62.67% and 33.33% for grade I and II respectively (Figure 1). None of the controls had grade III prostatomegaly. As table 1 depicts, this difference in the distribution of prostatomegaly grades was statistically significant (P=0.001).
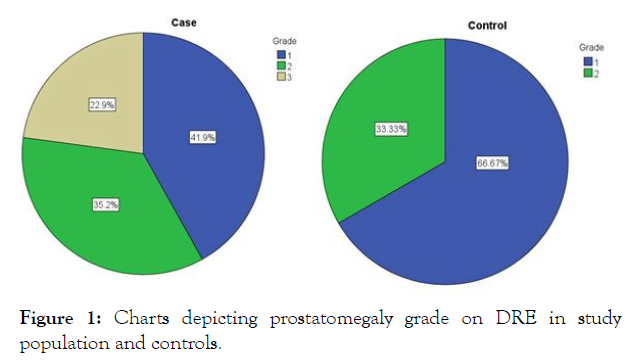
Figure 1: Charts depicting prostatomegaly grade on DRE in study population and controls.
International Prostate Symptom Score: As per table 1, for the ease of IPSS comparison, subjects were divided into two groups. Those with moderate to severe symptoms (score 9-35) and those with mild symptoms (score 0-8). The study population with vitamin D deficiency had significantly higher symptom score than controls. The difference was statistically significant on the Mann Whitney U test (P <0.001).
Binary logistic regression analysis showed a strong association between vitamin D deficiency in men with BPE and prostate volume greater than 20ml (OR 4.98, 95% CI 1.24 – 13.18, P=0.001) as well as between the vitamin D deficiency & moderate to severe IPSS (OR 4.22, 95% CI 2.16 – 14.76; P = 0.001). There was no direct association noted between serum PSA level and 25(OH) D levels in cases and controls (P > 0.05) (Table 2).
| Parameters | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Prostate volume >20 ml | 4.98 | 1.24 - 13.18 | 0.001* |
| Moderate to severe IPSS | 34.22 | 2.16- 14.76 | 0.001* |
Values are represented as numbers
*significant
Table 2: Association of Vitamin D deficiency (<20 ng/ml) with Prostate volume and IPSS on binarylogistic regression analysis.
Although ageing and the presence of androgens are known to be the most important factors in the pathogenesis of BPE, the exact mechanism is still ambivalent. Ageing-related changes in the skin, renal function, gut absorption, and reduced sunlight exposure may adversely impact the formation of vitamin D in elderly men[10]. The mean age of the study population, as well as controls, was comparable with other studies [11,12]. The median serum level of 25(OH) D in our participants was 18.95 ng/ml and vitamin D deficiency was detected in 70% of participants. We found that the mean prostate volume in vitamin D deficiency cases was 41.32 ml, which was significantly higher than 23.42 ml in controls. Our findings are complementary to the findings reported by Zhang, et al. [13]. They observed that the median prostate volume in vitamin D deficiency was 42 ml and in the control group it was 28 ml. In line with our study, an inverse correlation between serum 25-OH D level < 20 ng/ml and prostate volume was reported by Murphy, et al. [14]. Our study population was found to have significantly higher IPSS than controls. Incidence of moderate to severe IPSS was 78.6% in the study population, while it was 21.4% in controls. The difference was statistically significant. We found that vitamin D deficiency (level of <20 ng/ml) is associated with moderate to severe IPSS, on binary logistic regression. The findings of our study are consistent with those of Zhang, et al. [13] and Mohamed Elshazly, et al.[11] Unlike, Elshazly, et al. [11] we did not find any statistically significant association between serum PSA level and 25(OH) D level.
Our study was not devoid of limitations. Enlisting a few, we did not take into consideration age-specific PSA levels, instead, we used a PSA cut off <4 ng/ml. There is a possibility of seasonal variation of Vitamin-D level in our patients, which was not taken into consideration. Also, the study is cross-sectional & observational; hence we cannot establish a strong cause and effect association.
To conclude, this study was aimed to demonstrate the relationship of vitamin D deficiency with prostate volume and severity of IPSS in men with BPE. The men having BPE and vitamin D deficiency were found to have a greater prostate volume, higher prostatomegaly grade on DRE and higher IPSS in comparison to men with BPE and normal vitamin D levels. If further studies confirm this association, vitamin D deficiency may be used as a therapeutic target for preventing and treating BPE.
Citation: Kale S (2021) Can We Link Vitamin D Deficiency to Benign Prostatic Enlargement? An Observational Case Control Study. Med Surg Urol 10:264.
Received: 18-Aug-2021 Accepted: 17-Sep-2021 Published: 24-Sep-2021 , DOI: 10.35248/2168-9857.21.10.264
Copyright: © 2021 Kale S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.