
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2018) Volume 7, Issue 3
In sheep red blood cells (RBCs), considering osmotic fragility (OF) as an indicator, the reaction of monocarboxylic and dicarboxylic acids on membrane resistance to osmotic pressure were evaluated. Sheep RBCs were exposed to carboxylic acids at 0-100 mM in a buffer solution for 1 h and the 50% hemolysis was then determined by soaking in 0.1-0.8% NaCl solution. Although formic acid declined and n-caprylic acid increased OF, most of the monocarboxylic acids with straight hydrocarbon chains did not change OF in sheep RBCs. Whereas, all the tested dicarboxylic acids with straight hydrocarbon chains decreased OF with the degree of the decrease in OF dependent on the compound. Some monocarboxylic acids with branched or cyclic (including a benzene ring) hydrocarbon chains decreased OF with the degree of the decrease dependent on the number of carbons and form of branching in the hydrocarbon chain. Dicarboxylic acids with a cyclohexane ring or benzene ring decreased or tended to decrease OF with the degree of the decrease dependent on the position of the two carboxylic groups. There is no clear correlation between the effect of monocarboxylic or dicarboxylic acids on OF, and their partition coefficients. Thus the partition coefficient is not a suitable parameter for explaining the effect of both groups of carboxylic acid on the cell membrane as evaluated by the change in OF in sheep RBCs. It is speculated that the space composed of the acyl-chains of the phospholipids, into which hydrophobic hydrocarbons can enter to form a more rigid structure through their subsequent interaction, is an important factor related to the OF-decreasing effect of carboxylic acids.
Keywords: Carboxylic acid; Erythrocyte; Osmotic fragility; Membrane; Phospholipid; Partition coefficient; Sheep
Since mammalian red blood cells (RBCs) possess a basic cell membrane structure but no nucleus or complex system of the metabolism in the plasma, it has been used as an experimental model, particularly of the phospholipid bilayer of the cell membrane. There have been many reports that osmotic fragility (OF) in RBCs is a valuable tool for assessing the actions of various chemicals on the cell membrane in vitro. General [1] and local anesthetics [2], certain kinds of drugs [3] and toxins [4], inorganic [5] and organic compounds [6], as well as substances isolated from plants [7], and crude plant extracts [8], all induce changes in OF in the RBCs of various mammalian species.
It has been reported that the application of monocarboxylic acids carry a carboxylic base and a hydrocarbon chain of various lengths and structure (branched and cyclic chains, and benzene rings) increase OF in isolated rat RBCs [9-13]. These effects on OF in the RBCs occurred in a concentration-dependent manner for monocarboxylic acids and was also dependent on the number of carbons in the hydrocarbon chains in their moiety [9-13]. As the monocarboxylic acid-induced increase in OF was not eliminated by the pretreatment of rat RBCs with trypsin, it is clear that the surface protein in the RBC membrane is not involved in this increase in OF [9]. We consider that the monocarboxylic acids probably affect the phospholipid layer in the RBC membrane directly, resulting in a decrease in resistance to osmotic pressure. Unlike in rat RBCs, monocarboxylic acids possessing straight or cyclic hydrocarbon chains, including benzene rings, did not increase OF in guinea pig RBCs in vitro [12]. In contrast to monocarboxylic acids, dicarboxylic acids, which have two carboxylic groups bound to a hydrocarbon chain in the moiety, decreased OF in both rat [11,12] and guinea pig RBCs [12,14].
Permeation of monocarboxylic or dicarboxylic acids into the RBC membrane is thought to be necessary for inducing the effects on OF. The partition coefficient is a physicochemical parameter that reflects the hydrophobic/hydrophilic balance of chemical substances and is defined as the ratio of the concentrations of a substance between two solvents [15]. The octanol/water partition coefficient has been generally utilized as a pointer of the dispersion of hydrophobic drugs in cells, tissues and the body in general [16-18], as, in comparison to other non-polar solvents such as hexanol, cyclohexane, dodecane or chloroform, the value for n-octanol was reported to be closer to that of the phospholipids found in biological membranes [19]. We used the partition coefficient as an indicator and found on the basis of regression analysis that there is a positive and statistically significant correlation between the partition coefficient of monocarboxylic acids, but not dicarboxylic acids, and the degree of change in OF in rat RBCs [13]. Unlike in rat RBCs, there is no apparent correlation between the partition coefficient of either monocarboxylic or dicarboxylic acids and their effect on OF in guinea pig RBCs [14].
Through a series of experiments, we have also found that the OF response to carboxylic acids differs between rat and guinea pig RBCs [12]. It is suggested that interspecies differences exist for the RBC membrane response to carboxylic acids. We therefore analyzed the action of monocarboxylic and dicarboxylic acids on OF in isolated sheep RBCs in this study.
Our objective was to clarify the chemical structures of carboxylic acids and their derivatives required to change OF in sheep RBCs. In addition, some explanation of the differences in OF response in RBCs among the species is required, particularly in relation to the differences in RBC membrane characteristics. We examined the effect of carboxylic acids on OF in sheep RBCs using various monocarboxylic and dicarboxylic acids, and isomers of those carboxylic acids and derivatives, including chemicals possessing cyclic hydrocarbon chains. In the present experiment, these structure-activity relationships clarified the chemical structure needed for changing OF in sheep RBCs and how carboxylic molecules affect the lipid bilayer in a cell membrane to change OF. In addition, these evaluations also afforded a key to explain the differences in OF response in RBCs to carboxylic acids among different animal species.
Reagents
Biochemical grade formic acid, acetic acid, propionic acid, n-butyric acid, n-valeric acid, n-caproic acid, n-enanthic acid, n-caprylic acid, isobutyric acid, isovaleric acid, 2-methyl-butyric acid, dimethylpropionic acid, 2-methyl-n-valeric acid, 3-methyln-n-valeric acid, 4-methyl-n-valeric acid, 2-ethyl-n-butyric acid, 3,3-dimethyl-n-butyric acid, cyclopropane-carboxylic acid, cyclobutane-carboxylic acid, cyclopentane-carboxylic acid, cyclohexane-carboxylic acid, benzoic acid (benzene-carboxylic acid), oxalic acid, malonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid, suberic acid, azelaic acid, and 1, 2-, 1, 3-, and 1, 4-cyclohexane-dicarboxylic acids (cis- and transmixture), 1, 2-benzene-dicarboxylic acid (phthalic acid), 1, 3-benzenedicarboxylic acid (isophthalic acid), 1, 4-benzene-dicarboxylic acid (terephthalic acid) were purchased from Tokyo Kasei Kogyo Co., Ltd (Tokyo, Japan) or Wako Pure chemical Co., Ltd. (Osaka, Japan). All other reagents used in this study were of biochemical grade.
Preparation of sheep RBCs
Mongrel female sheep aged 3 to 5 years old (n=20, body weight 70-85 kg) fed in the Ecorin Village Farm (Eniwa, Hokkaido) were used for experiments. A pelleted diet and hay as well as tap water were accessed freely by the animals. Blood samples from the sheep were collected by a veterinarian and purchased from the Ecorin Village Farm. The sampling and treatment of blood were performed as follows. Blood samples (30 ml) were collected from the left jugular vein into a heparinized test tube through a hypodermic needle. The blood samples were transported from the farm to the laboratory of the Hokkaido Bunkyo University and then kept in refrigerator at 4°C for about 18 hours. RBCs were separated from plasma and buffy coat by aspiration after centrifugation at 2000 g for 15 min (Model 2420, Kubota Inc., Tokyo, Japan). The crude RBCs thus obtained were then washed three times with two volumes of cold 0.9% NaCl solution. A thick stuffed cell suspension was gotten and from that point kept in ice-cold water until subsequent treatment.
Experimental procedure
The experimental procedures followed those in our previous report [11]. Briefly, the packed cell suspension described above (30 μl) was transported into 0.6 ml of phosphate-NaCl buffer solution (pH 7.4) containing each of the carboxylic acids or their derivatives at 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50 or 100 mM in 1.5-ml micro test tubes (Nichiryo Co., Ltd., Tokyo, Japan). An appropriate amount of NaCl was added to the buffer solution to adjust the osmolarity for each chemical tested. The RBC suspensions applied to the chemical substances were incubated by shaking (1 stroke/sec) at 37°C for 1 h (Shaking Bath TBK 202 DA, Advantec Co., Ltd., Tokyo, Japan). Each RBC suspension was gently mixed by a mixer (Vortex Genie 2, Model-G560, Scientific Industry, Inc. NY., USA) following incubation, and 50 μl of each suspension was transferred into a 96-deep-well microplate (2 ml volume, Whatman Inc., Piscataway, NJ, USA) containing a 1 ml NaCl solution ranging from 0.1 to 0.8%. The deep-well microplate was instantly centrifuged at 1300 g (Plate Spin II, Kubota Inc., Tokyo, Japan) for 10 min at room temperature. The supernatants (200 μl) containing various concentrations of hemoglobin derived from the burst RBCs were transferred into another 96-well microplate (300 μl volume, Whatman Inc., Piscataway, NJ, USA) and were determined colorimetrically at 540 nm (Microplate Reader Model 680, Bio-Rad Laboratories, Tokyo, Japan).
Statistical analysis
Complete hemolysis of RBC suspension is induced in 0.1% NaCl solution, so that the hemoglobin concentration is defined as 100%. When the hemolysis of RBCs is not present in the 0.8 % NaCl solution, the hemoglobin concentration is defined as 0%. The effective concentration of the NaCl solution inducing 50% hemolysis (EC50) of the applied RBCs was calculated from the hemolysis curve by using a straight-line equation between the points immediately adjacent to 50%. OF in the RBCs was expressed as the EC50 value. All values are expressed as means ± S.D. (n=6). The significance of the differences between the control (0 mM) and subsequent concentrations (0.1-100 mM) was calculated by Dunnett's test following one-way ANOVA. As obvious changes in OF were obtained by the application of most carboxylic acids at 10, 25, 50 and 100 mM, the differences from the control value at 0 mM were calculated and expressed as ΔEC50 (NaCl%). The partition coefficients of the substances examined in this experiment were mainly quoted from the PubChem or the ChemSpinder websites for chemical and physical properties [20,21]. The relationship between the partition coefficient of each carboxylic acid and the ΔEC50 of the RBCs was confirmed by regression analysis. Statistical analyses were performed using Excel Tokei for Windows 2012 (SSRI Co., Ltd., Tokyo, Japan). Statistical significance was set at a P value <0.05 or 0.01.
Effects of monocarboxylic acids with a straight hydrocarbon chain
Typical concentration-response relationships between monocarboxylic acids having straight hydrocarbon chains (acetic, n-valeric and n-caprylic acids) and OF are shown in Figure 1. Although the use of acetic (C1, number of carbons in the hydrocarbon element) and n-valeric (C4) acids did not effect OF at any of the examined concentrations from 0.1 to 100 mM, n-caprylic acid (C7) only increased OF in sheep RBCs at 100 mM (P<0.05). Monocarboxylic acids with straight chain hydrocarbons of 1 to 6 carbons in length chain did not have any change on OF in sheep RBCs (Table 1). Although formic acid (C0) at concentrations of 25 to 100 mM decreased OF (P<0.01), n-caprylic acid (C7) only increased OF (P<0.01) in sheep RBCs at 100 mM as mentioned above.
Figure 1:Effects of monocarboxylic and dicarboxylic acids with straight hydrocarbon chains on OF in sheep RBCs. The effects of acetic and malonic acid (A), n-valeric and adipic acid (B), and n-caprylic and azelic acid (C) on OF are presented. Values are the means ± SD (n=6). The monocarboxylic acids are represented by closed circles (●) and the dicarboxylic acids by closed triangles (▲) on each panel. Open symbols indicate that there was a significant difference between the control (0 mM) and subsequent concentrations (0.1-100 mM) based on Dunnett’s test (P <0.05 including 0.01).
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) |
|---|---|---|---|---|
| 0 | Formic acid | -0.54 | 10 | -0.014 ± 0.009 |
| H-COOH | 25 | -0.045 ± 0.012 ** | ||
| 50 | -0.054 ± 0.010 ** | |||
| 100 | -0.075 ± 0.022 ** | |||
| 1 | Acetic acid | -0.17 | 10 | 0.003 ± 0.014 |
| CH3-COOH | 25 | -0.005 ± 0.012 | ||
| 50 | -0.012 ± 0.014 | |||
| 100 | -0.018 ± 0.024 | |||
| 2 | Propionic acid | 0.33 | 10 | 0.000 ± 0.009 |
| CH3-CH2-COOH | 25 | -0.010 ± 0.014 | ||
| 50 | -0.013 ± 0.020 | |||
| 100 | -0.008 ± 0.037 | |||
| 3 | n-Butyric acid | 0.79 | 10 | -0.001 ± 0.017 |
| CH3-(CH2)2-COOH | 25 | 0.000 ± 0.022 | ||
| 50 | -0.010 ± 0.020 | |||
| 100 | -0.020 ± 0.044 | |||
| 4 | n-Valeric acid | 1.39 | 10 | 0.001 ± 0.013 |
| CH3-(CH2)3-COOH | 25 | 0.002 ± 0.016 | ||
| 50 | 0.002 ± 0.016 | |||
| 100 | 0.007 ± 0.019 | |||
| 5 | n -Caproic acid | 1.92 | 10 | 0.012 ± 0.014 |
| CH3-(CH2)4-COOH | 25 | -0.003 ± 0.019 | ||
| 50 | 0.011 ± 0.015 | |||
| 100 | 0.010 ± 0.026 | |||
| 6 | n-Enantic acid | 2.42 | 10 | 0.003 ± 0.015 |
| CH3-(CH2)5-COOH | 25 | 0.002 ± 0.010 | ||
| 50 | 0.000 ± 0.008 | |||
| 100 | -0.018 ± 0.033 | |||
| 7 | n-Capric acid | 3.05 | 10 | 0.002 ± 0.007 |
| CH3-(CH2)6-COOH | 25 | -0.006 ± 0.006 | ||
| 50 | -0.009 ± 0.010 | |||
| 100 | 0.065 ± 0.033 ** |
Table 1: Monocarboxylic acids possessing straight hydrocarbon chains, their chemical structure, partition coefficients and effect on OF in sheep RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [20] or ChemSpider [21] website. Asterisks (* and **) indicate that there was a significant difference (P <0.05 and P <0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Effects of dicarboxylic acids with a straight hydrocarbon chain
Typical concentration-response relationships between dicarboxylic acids having straight hydrocarbon chains (malonic, adipic and azelaic acids) and OF are shown in Figure 1. The application of malonic (C1), adipic (C4) and azelaic (C7) acids decreased OF in a dose-dependent manner (P<0.05). All of the examined dicarboxylic acids having a straight hydrocarbon chain of 0 to 8 carbons in length decreased OF in sheep RBCs (Table 2). The five dicarboxylic acids from succinic acid (C2) to suberic acid (C6) decreased OF in a dose-dependent manner (P<0.05 or 0.01). Both oxalic acid (C0) and malonic acid (C1) also decreased OF dose-dependently (P <0.05 or 0.01). The degree of the decrease in OF induced by oxalic or malonic acid was greater than that by the five dicarboxylic acids. Azelaic (C7) and sebacic (C8) acids also lowered OF dose-dependently (P <0.05 or 0.01) with the OF values decreasing in the order shown. The OF values induced by these two dicarboxylic acids were lower than those induced by five dicarboxylic acids mentioned above.
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) |
|---|---|---|---|---|
| 0 | Oxalic acid | -0.81 | 10 | -0.025 ± 0.014 |
| HOOC-COOH | 25 | -0.041 ± 0.014 | ||
| 50 | -0.090 ± 0.039 ** | |||
| 100 | -0.131 ± 0.066 ** | |||
| 1 | Malonic acid | -0.81 | 10 | -0.018 ± 0.024 |
| HOOC-CH2-COOH | 25 | -0.043 ± 0.015 ** | ||
| 50 | -0.072 ± 0.011** | |||
| 100 | -0.112 ± 0.016 ** | |||
| 2 | Succinic acid | -0.59 | 10 | -0.007 ± 0.011 |
| HOOC-(CH2)2-COOH | 25 | -0.020 ± 0.011 | ||
| 50 | -0.036 ± 0.019 | |||
| 100 | -0.056 ± 0.025 ** | |||
| 3 | Glutaric acid | -0.29 | 10 | -0.011 ± 0.018 |
| HOOC-(CH2)3-COOH | 25 | -0.009 ± 0.014 | ||
| 50 | -0.038 ± 0.034 | |||
| 100 | -0.058 ± 0.024 ** | |||
| 4 | Adipic acid | 0.08 | 10 | -0.003 ± 0.013 |
| HOOC-(CH2)4-COOH | 25 | -0.015 ± 0.011 | ||
| 50 | -0.026 ± 0.015 | |||
| 100 | -0.059 ± 0.030 * | |||
| 5 | Pimeric acid | 0.61 | 10 | -0.005 ± 0.015 |
| HOOC-(CH2)5-COOH | 25 | -0.011 ± 0.011 | ||
| 50 | -0.025 ± 0.023 | |||
| 100 | -0.050 ± 0.016 * | |||
| 6 | Suberic acid | 0.8 | 10 | -0.010 ± 0.012 |
| HOOC-(CH2)6-COOH | 25 | -0.017 ± 0.017 | ||
| 50 | -0.033 ± 0.014 | |||
| 100 | -0.062 ± 0.021 ** | |||
| 7 | Azelaic acid | 1.57 | 10 | 0.001 ± 0.010 |
| HOOC-(CH2)7-COOH | 25 | -0.012 ± 0.012 | ||
| 50 | -0.034 ± 0.026 | |||
| 100 | -0.071 ± 0.022 ** | |||
| 8 | Sebacic acid | 2.2 | 10 | -0.019 ± 0.013 |
| HOOC-(CH2)8-COOH | 25 | -0.054 ± 0.016 * | ||
| 50 | -0.087 ± 0.011 ** | |||
| 100 | -0.119 ± 0.034 ** |
Table 2: Dicarboxylic acids possessing straight hydrocarbon chains, their chemical structure, partition coefficients and effect on OF in sheep RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [20] or ChemSpider [21] website. Asterisks (* and **) indicate that there was a significant difference (P <0.05 and P <0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Effects of monocarboxylic acids with a branched hydrocarbon chain
Typical concentration-response relationships between monocarboxylic acids having branched hydrocarbon chains (2-methylbutyric, dimethyl-propionic, 2-methyl-n-valeric, 3-methyl-n-valeric, 2-ethyl-n-valeric and 3,3-dimethyl-n-butyric acids) and OF are shown in Figure 2. The effects of those 6 branched-chain monocarboxylic acids (C4 or C5) on OF in sheep RBCs depended on the form of the branched hydrocarbon. Table 3 shows the changes in OF induced by all tested branched chain monocarboxylic acids. As with its parent compound, isobutyric acid (C3), a branched isomer of n-butyric acid, did not have any effect on OF in sheep RBCs. Among the isomers of n-valeric acid (C3), isovaleric acid and dimethyl-propionic acid, but not 2-methy butyric acid, decreased OF in a dose-dependent manner (P <0.05 or 0.01). Three branched isomers of n-caproic acid (C6), 2-methyl-nvaleri acid, 4-methyl-n-valeric acid and 3,3-dimetyl-n-butyric acid, decreased OF dose-dependently (P <0.05 or 0.01). Two other n-caproic acid (C6) isomers, 3-metyl-n-varelic acid and 2-eithyl-nbutylic acid, did not have any effect on OF in sheep RBCs.
Figure 2:Effects of monocarboxylic acids with branched hydrocarbon chains on OF in sheep RBCs. The effects of isomers of n-valeric acid (A): 2-methylbutyric (●) and dimethyl-propionic acid (▲), isomers of n-caprylic acid (B): 2-methyl- (●) and 3metyl-n-valeric acid (▲), and (C): 2-ethyl-n-valeric (●) and 3,3-dimethyl-n-butyric acid are presented. Values are the means ± SD (n = 6). Open symbols indicate that there was a significant difference between the control (0 mM) and subsequent concentrations (0.1-100 mM) based on Dunnett’s test (P <0.05 including 0.01).
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 3 | iso-Butyric acid | 0.94 | 10 | -0.011 ± 0.011 | |
 |
25 | -0.018 ± 0.008 | |||
| 50 | -0.014 ± 0.017 | ||||
| 100 | -0.004 ± 0.014 | ||||
| 4 | iso-Valeric acid | 1.16 | 10 | -0.016 ± 0.013 | |
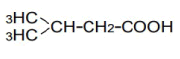 |
25 | -0.023 ± 0.008 | |||
| 50 | -0.045 ± 0.015 | ** | |||
| 100 | -0.053 ± 0.015 | ** | |||
| 4 | 2-Methyl-butyric acid | 1.18 | 10 | 0.000 ± 0.003 | |
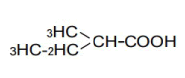 |
25 | -0.010 ± 0.018 | |||
| 50 | -0.009 ± 0.011 | ||||
| 100 | -0.015 ± 0.016 | ||||
| 4 | Dimetyl-propionic acid | 1.48 | 10 | -0.016 ± 0.022 | |
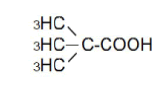 |
25 | -0.027 ± 0.029 | |||
| 50 | -0.039 ± 0.034 | * | |||
| 100 | -0.089 ± 0.015 | ** | |||
| 5 | 2-Mrthy-n-valeric acid | 1.80 | 10 | -0.015 ± 0.007 | |
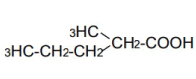 |
25 | -0.034 ± 0.014 | * | ||
| 50 | -0.057 ± 0.011 | ** | |||
| 100 | -0.092 ± 0.007 | ** | |||
| 5 | 3-Mrthy-n-valeric acid | 1.56 | 10 | -0.010 ± 0.012 | |
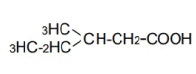 |
25 | -0.003 ± 0.018 | |||
| 50 | -0.001 ± 0.025 | ||||
| 100 | -0.009 ± 0.023 | ||||
| 5 | 4-Mrthy-n-valeric acid | 1.66 | 10 | -0.004 ± 0.006 | |
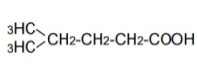 |
25 | -0.016 ± 0.009 | |||
| 50 | -0.033 ± 0.006 | * | |||
| 100 | -0.056 ± 0.051 | ** | |||
| 5 | 2-ethyl-n-butyric acid | 1.66 | 10 | 0.005 ± 0.011 | |
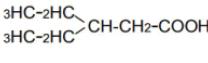 |
25 | -0.005 ± 0.015 | |||
| 50 | 0.000 ± 0.014 | ||||
| 100 | -0.004 ± 0.017 | ||||
| 5 | 3,3-Dimethyl-n-butyric acid | 1.47 | 10 | -0.019 ± 0.024 | |
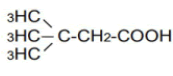 |
25 | -0.034 ± 0.021 | |||
| 50 | -0.047 ± 0.020 | * | |||
| 100 | -0.072 ± 0.028 | ** | |||
Table 3: Monocarboxylic acids possessing branched hydrocarbon chains, their chemical structure, partition coefficients and effect on OF in sheep RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [20] or ChemSpider [21] website. Asterisks (* and **) indicate that there was a significant difference (P <0.05 and P <0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Effects of monocarboxylic acids with a cyclic hydrocarbon chain or benzene ring
Typical concentration-response relationships between monocarboxylic acids having cyclic hydrocarbon chains (cyclopropane, cyclopentane and cyclohexane carboxylic acids and benzoic acid) and OF are shown in Figure 3. Cyclopentane carboxylic acid (C5), but not cyclopropane carboxylic acid (C3), slightly decreased OF at 100 mM. Although cyclohexane carboxylic acid (C6) did not effect OF, benzoic acid (C6), which possesses a benzene nucleus composed of conjugated double bonds, decreased OF in a dose-dependent manner in sheep RBCs (P <0.05). Table 4 shows the changes in OF induced by all of the tested cyclic-hydrocarbon chain monocarboxylic acids. Among the 4 monocarboxylic acids (C3-C6) possessing cyclic hydrocarbons, only cyclopentane carboxylic acid (C5) decreased OF dose-dependently in sheep RBCs (P <0.05 or 0.01). Benzoic acid (C6) also decreased OF in a dose-dependent manner (P <0.05 or 0.01).
Figure 3:Effects of monocarboxylic and dicarboxylic acids with cyclic hydrocarbon chains on OF in sheep RBCs. The effects of cyclobutane (●) and cyclopentane monocarboxylic acids (▲) (A), cyclohexane monocarboxylic acid (●) and 1,2-cyclohexane- dicarboxylic acid (▲) (B), and benzoic acid (●) and phthalic acid (▲) (C) on OF are presented. Values are the means ± SD (n=6). Open symbols indicate that there was a significant difference between the control (0 mM) and subsequent concentrations (0.1-100 mM) based of Dunnett’s test (P <0.05 including 0.01).
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) |
|---|---|---|---|---|
| 3 | Cyclopropane-carboxylic acid | 0.08 | 10 | 0.000 ± 0.010 |
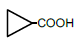 |
25 | -0.005 ± 0.014 | ||
| 50 | 0.004 ± 0.010 | |||
| 100 | 0.011 ± 0.014 | |||
| 4 | Cyclobutane-carboxylic acid | 0.65 | 10 | -0.011 ± 0.011 |
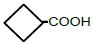 |
25 | -0.021 ± 0.012 | ||
| 50 | -0.027 ± 0.011 | |||
| 100 | -0.035 ± 0.014 | |||
| 5 | Cyclopentane-carboxylic acid | 1.21 | 10 | -0.007 ± 0.011 |
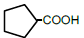 |
25 | -0.010 ± 0.005 | ||
| 50 | -0.022 ± 0.018 | |||
| 100 | -0.046 ± 0.014** | |||
| 6 | Cyclohexane-monocarboxylic acid | 1.96 | 10 | 0.007 ± 0.010 |
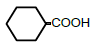 |
25 | 0.000 ± 0.016 | ||
| 50 | -0.012 ± 0.021 | |||
| 100 | -0.026 ± 0.026 | |||
| 6 | Benzoic acid | 1.87 | 10 | -0.007 ± 0.020 |
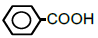 |
25 | -0.030 ± 0.034 | ||
| 50 | -0.052 ± 0.034* | |||
| 100 | -0.081 ± 0.024** |
Table 4: Monocarboxylic acids possessing cyclic hydrocarbon chains, their chemical structure, partition coefficients and effect on OF in sheep RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [20] or ChemSpider [21] website. Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data
for 10, 25, 50 and 100 mM are presented.
Effects of dicarboxylic acids with a cyclic hydrocarbon chain or benzene ring
Typical concentration-response relationships between monocarboxylic acids having cyclic hydrocarbon chains (C6) and OF are shown in Figure 3. 1,2- Cyclohexane-dicarboxylic acid and phthalic acid decreased OF dose-dependently (P <0.05). Table 5 shows the changes in OF induced by all the tested cyclic-hydrocarbon chain dicarboxylic acids (C6). 1,2- and 1,3-, but not 1,4-, cyclohexanedicarboxylic acids dose-dependently decreased OF (P <0.01), with similar value for ΔEC50. While three isomers of phthalic acid also decreased OF dose-dependently (P <0.05 or 0.01), the effect of these isomers depended on the position of two carboxylic groups on the benzene ring.
| No of hydrocarbon | Carboxylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) |
|---|---|---|---|---|
| 6 | 1,2-Cyclohexane-dicarboxylic acid | 0.64 | 10 | -0.002 ± 0.012 |
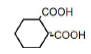 |
25 | -0.006 ± 0.009 | ||
| 50 | -0.016 ± 0.010 | |||
| 100 | -0.045 ± 0.015** | |||
| 6 | 1,3-Cyclohexane-dicarboxylic acid | 0.46 | 10 | 0.005 ± 0.017 |
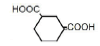 |
25 | -0.003 ± 0.020 | ||
| 50 | -0.007 ± 0.007 | |||
| 100 | -0.019 ± 0.015 | |||
| 6 | 1,4-Cyclohexane-dicarboxylic acid | 0.83 | 10 | -0.004 ± 0.008 |
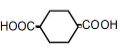 |
25 | -0.012 ± 0.011 | ||
| 50 | -0.018 ± 0.014 | |||
| 100 | -0.040 ± 0.031 ** | |||
| 6 | Phthalic acid | 1.73 | 10 | -0.016 ± 0.008 |
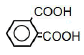 |
25 | -0.029 ± 0.025* | ||
| 50 | -0.071 ± 0.018** | |||
| 100 | -0.123 ± 0.018** | |||
| 6 | Isophathalic acid | 1.66 | 10 | 0.011 ± 0.008 |
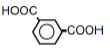 |
25 | -0.009 ± 0.018 | ||
| 50 | -0.019 ± 0.015 | |||
| 100 | -0.030 ± 0.021 * | |||
| 6 | Terephthalic acid | 2.00 | 10 | 0.015 ± 0.011 |
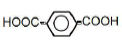 |
25 | -0.009 ± 0.007 | ||
| 50 | -0.022 ± 0.015 | |||
| 100 | -0.048 ± 0.033** |
Table 5:Dicarboxylic acids possessing cyclic hydrocarbon chains, their chemical structure, partition coefficients and effect on OF in sheep RBCs in vitro. Values are means ± SD (n=6). The partition coefficients were obtained from the PubChem [20] or ChemSpider [21] website. Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) based on the Dunnett’s test. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Relationship between partition coefficients and changes in OF
Regression analysis was used to determine the connection between the partition coefficient of each monocarboxylic or dicarboxylic acid applied at 10, 25, 50 or 100 mM, and their respective effects on OF as indicated by the ΔEC50 value. Table 6 shows correlation efficient “r” and statistical significance “P” of these analyses. There were no definite statistically important interaction between the partition coefficients of either monocarboxylic or dicarboxylic acids, and their respective effects on OF in sheep RBCs at concentrations from 10 to 100 mM.
| Substances | Dose (mM) | Regression analysis | |
|---|---|---|---|
| r value | P value | ||
| Monocarboxylic acids 22 substances | 10 | 0.3547 | 0.1052 |
| 25 | 0.2888 | 0.1923 | |
| 50 | 0.2796 | 0.2075 | |
| 100 | 0.3912 | 0.0718 | |
| Dicarboxylic acids 15 substanes | 10 | 0.424 | 0.1152 |
| 25 | 0.1425 | 0.6123 | |
| 50 | 0.2162 | 0.439 | |
| 100 | 0.2083 | 0.4563 | |
Table 6: Correlation between the partition coefficients of carboxylic acids and change in EC50 during hemolysis in sheep RBCs. Values were calculated by regression analysis (mean value of each carboxylic acid; n=6) between the partition coefficients and changes in EC50 during hemolysis induced by each dose of the monocarboxylic and dicarboxylic acids. Correlation efficient “r” and significance “P” are shown. P <0.05 is defined as statistically significant in the present study.
The application of monocarboxylic acids having straight hydrocarbon chains (C1-C6), except for formic (C0) and n-caprylic acid (C7), did not affect the OF in the sheep RBCs. Although formic acid (C0) decreased OF in a dose-dependent manner, n-caprylic acid (C7) increased OF only at 100 mM. On the contrary, the effects on OF induced by the monocarboxylic acids with branched hydrocarbon chains (C4-C6) were dependent on the structure of the hydrocarbon branches. Among the monocarboxylic acids with branched hydrocarbon chains, isovaleric (C5), dimethyl-propionic (C5), 2-methyl-n-valeric (C6), 4-methyl-n-valeric (C6) and 3,3-dimetyln- butylic acid (C6) have the ability to decrease the OF in a dosedependent manner. Among the monocarboxylic acids with cyclic hydrocarbons, cyclopentane-carboxylic (C5) and benzoic acid (C6) decreased OF dose-dependently, whereas the other monocarboxylic acids with cyclic hydrocarbon chains (C3, C4 and C6) does not change the OF in sheep RBCs. In contrast, all tested dicarboxylic acids with straight hydrocarbon chains (C0-C7) decreased OF dose-dependently, but the degree of the decrease in OF was dependent on the compound. Although 1,2- and 1,4-cyclohexane dicarboxylic acids (C6) decreased OF in a dose-dependent manner, 1,3-cyclohexane dicarboxylic acid did not change OF at any of the concentrations tested. All three isomers of phthalic acid (C6) also decreased OF and the degree of the effect on OF depended on the positions of the two carboxylic groups on the benzene ring.
No clear relationship is present between the partition coefficients of either monocarboxylic acids or dicarboxylic acids and their effects on OF by regression analysis. Although the partition coefficient can be used as an indicator of the permeation of substances into the cell membrane [22-25], the results of the regression analysis indicates that the partition coefficient cannot be used as a parameters for the evaluation of the effect of both types of carboxylic acids on OF in sheep RBCs.
We have already reported that, based on the same experimental procedure as that in this report, most monocarboxylic acids with straight, branched or cyclic hydrocarbon chains, including benzene rings, increase OF in rat RBCs [10-13]. On the contrary, most of the monocarboxylic acids did not alter or actually reduced OF in guinea pig RBCs [12-14]. A positive and statistically significant correlation between the partition coefficients of the monocarboxylic acids and the OF responses induced by monocarboxylic acids in the rat RBCs can be observed [13], but not in the guinea pig RBCs [14]. Due to absence of OF response to monocarboxylic acids, the OF response to monocarboxylic acids in sheep RBCs closely resembles that in guinea pig RBCs, but not rat RBCs. These results shows that the partition coefficient of monocarboxylic acid cannot able be used as a common parameter for estimating the degree of influence on OF in the RBC membrane in different animal species other than rats.
Although most of the dicarboxylic acids decreased OF both in the rat and guinea pig RBCs [11-14], there was no clear relationship between the partition coefficients of the dicarboxylic acids and the OF responses induced by these acids in the RBCs of these species [13,14]. In this respect, there is no difference in OF response to dicarboxylic acids among the rat, guinea pig and sheep RBCs. The results of our series of experiment show that the partition coefficient of dicarboxylic acid also cannot be used as a common parameter for estimating the degree of influence on OF in the RBC membrane.
The partition coefficient is a physicochemical parameter indicating the hydrophobic/hydrophilic balance of chemical substances between two solvents [15]. The partition coefficient is used as an indicator of the permeation of the substance into the cell membrane [26-29]. In small molecules, the partition coefficient is directly proportional to the length of hydrocarbon chain and it generally increases with increases in the length of hydrocarbon chains, or the number of carbons in the hydrocarbonmoiety [30,31]. The monocarboxylic acids having straight hydrocarbon chains used in the present experiment were also reported to show increased permeation into the membrane with increases in the number of carbons in the hydrocarbons [32].
The octanol/water partition coefficient has been widely used as an indicator of the distribution of hydrophobic drugs in cells, tissues and the body in general [16-18]. There are many cases in which we cannot recognize the actions of chemicals on biological or artificial phospholipid membranes as they did not correspond to the partition coefficient of each substance [33-35]. The reason is not only due the chemical properties of the substances, such as shape, dimensions and ionization status, but also due to membrane, such as the phospholipid head type, form and length of the acyl-chain and amount of cholesterol contained in the cell membrane [36-41].
With regard to the difference in the effects of monocarboxylic acids on OF between the rat [10-12,14], guinea pig [12,14] and sheep RBCs, we speculate that differences in the phospholipid composition of the RBC membrane contributes, at least in part, to the differences in OF response. Phospholipids in the RBC membrane are composed of a hydrophilic head containing phosphorus and two hydrophobic acylchains derived from fatty acids. There are various kinds of head element among the phospholipids (PC: Phosphatidyl choline, PE: Phosphatidyl ethanolamine, PS: Phosphatidyl serine, PI: Phosphatidyl inositol, SM: Sphingomyelin etc.) in the RBC membrane and the ratio of head types is reported to differ among RBCs from different mammals [42]. Although it was reported that the composition of the head group affect the fluidity of the RBC membrane [43], the phospholipid fluidity index (PFI = PC/(PE + SM)) calculated from the ratio of head groups dose not differ markedly between the rat and guinea pig RBC membranes [44]. There is one report comparing the composition of the head groups of phospholipids among rat, guinea pig and sheep RBCs [45]. Although the PC value was not obtained for sheep RBCs in this report [45], there are two reports indicating that PC, PE and SM values could only be determined in sheep RBCs [46,47]. The PFI value calculated for sheep RBCs was markedly lower than those for rat and guinea pig RBCs due to the high percentage of PE in the sheep RBCs compared to those in rat and guinea pig RBCs (Table 8). Thus, it is impossible to explain the differences in OF response to monocarboxylic acids between the rat, guinea pig and sheep RBCs based on the PFI previously presented [44].
On the other hand, there are no reports comparing the fatty acids compositions among the rat, guinea pig and sheep RBC membrane based on data from the same laboratory. The fatty acids compositions in the phospholipid layers vary considerably between the rat and guinea pig RBCs [48], and between the rat sand sheep RBCs [49] in different two reports. We calculated the saturation index (SI = Stearic acid / Oleic acid), which indicates cell membrane fluidity [50], and found that whereas SI in the rat RBCs was lower than that in guinea pig RBCs, that in the sheep RBCs was markedly lower than that in the rat RBCs. It, therefore, appears impossible to use the SI value from previous research [50] to explain the differences in the actions of monocarboxylic acids on OF in the RBCs among rats, guinea pigs and sheep (Table 7). On the other hand, the most common fatty acid in rat RBCs is arachidonic acid, with a percentage of up to approximately 30 or 22% of the total fatty acids [48,49]. In contrast, the contribution of arachidonic acid the total fatty acids in guinea pig and sheep RBCs is 18 and 1.5%, respectively [48,49]. In this respect, fatty acid composition in the rat RBC is distinct from that in guinea pig and sheep RBCs (Table 8).
| Rat | Guinea pig | Sheep | Sheep | Sheep | |
|---|---|---|---|---|---|
| Phosphatidyl choline (PC) | 47.1 | 41.1 | - | 11.03 | 14.32 |
| Phosphatidyl ethanolamine (PE) | 21.5 | 24.6 | 26.2 | 1.58 | 3.46 |
| Phosphatidyl serine (PS) | 10.8 | 16.8 | 14.1 | 4.04 | 6.22 |
| Phosphatidyl inositol (PI) | 3.5 | 2.4 | 2.9 | 3.44 | 2.72 |
| Sphingomyelin (SM) | 12.8 | 11.1 | 51 | 65.92 | 62.28 |
| Phosphatidic acid (PA) | < 0.3 | 4.2 | < 0.3 | - | - |
| Phosphatidyl glycerol (PG) | - | - | - | 13.98 | 11.24 |
| Lysophospadidyl choline (LC) | 3.8 | < 0.3 | - | - | - |
| Unidetified | - | - | 4.8 | - | - |
| Phospholipid fluidity index (PC/(PE+SM)) | 1.4 | 1.2 | - | 0.16 | 0.22 |
Table 7: Phospholipid composition and phospholipid fluidity index in rat, guinea pig and sheep RBC membranes. Values are the percentage of total phospholipids in the RBC membrane. The values are quoted from A [45], B [46] and [47]. The phospholipid fluidity index was calculated from the respective phospholipid values using the formula (PC/(PE + SM)) [43].
| Fatty acid and saturaion index | A | B | |||
|---|---|---|---|---|---|
| Rat | Guinea pig | Rat | Sheep | ||
| Saturated fatty acid | |||||
| Palmitic acid | (16:0) | 22.1 | 12 | 25.1 | 9 |
| Stearic acid | (18:0) | 14.8 | 24.9 | 15.1 | 13.6 |
| Lignoceric acid | (24:0) | - | - | 0.8 | 1.3 |
| Unsatturaed fatty acid | |||||
| Oleic acid | (18:1) | 8.9 | 9.9 | 11.5 | 53.6 |
| Linoleic acid | (18:2) | 11.4 | 19.9 | 11 | 7.8 |
| γ-Linolenic acid | (18:3) | - | - | 0.5 | 1.8 |
| Dihomo-γ-linolenic acid | (20:3) | 0.8 | 1.3 | Tr | Tr |
| Arachidonic acid | (20:4) | 30 | 18 | 22.2 | 1.5 |
| Eicosapentaenoic acid | (20:5) | 0.3 | 1.8 | 1.9 | 0.6 |
| Docosatetraenoic acid | (22:4) | 0.8 | - | Tr | Tr |
| Docosapentaenoic acid | (22:5) | 0.6 | 3.8 | 3.4 | Tr |
| Docosahexaenoic acid | (22:6) | 5.2 | 0.6 | 4.4 | - |
| Nervonic acid | (24:1) | - | - | Tr | 8.7 |
| Saturation index (Saturated/Unsatturaed) | 0.63 | 0.7 | 0.75 | 0.32 | |
| Saturation index (Stearic/Oleic) | 1.7 | 2.5 | 1.3 | 0.3 | |
Table 8: Fatty acids composition in the phospholipid and saturation index in rat, guinea pig and sheep RBC membranes. Values are the percentage of total phospholipids in the RBC membrane. The values are quoted from A [48] and B [49]. The saturation index was calculated from the respective fatty acid values using the formula (Stearic acid/Oleic acid) [51].
Arachidonic acid is a polyunsaturated fatty acids with a crooked hydrocarbon chain due to four unsaturated carbon bonds in its moiety. This molecular structure is thought to irritate the inflexible binding of acyl-chains aligned straightly in the phospholipid layer of the cell membrane (Figure 4). In a previous experiment, total replacement of native PC with 1-palmitoil-2-oleoyl and 1-palmitoil-2-linoleoyl PC in the outer layer of human erythrocytes by using specific PC transfer protein did not have any effect on OF values or potassium ion (K+) permeability [51]. On the other hand, the introduction of 1-palmitoil- 2-alachidonoyl PC into human RBCs induces K+ leakage from cells [51]. In cell membrane with a high proportion of arachidonic acid such as rat RBCs, monocarboxylic acids with long hydrocarbon chains and high partition coefficients are speculated to invade the acyl-chain matrix, which has loose bonds and a certain amount of free space, in the phospholipid layer (Figure 4). Monocarboxylic acids with longer hydrocarbon chains, but not those with shorter hydrocarbon chains, have a stronger effect on the RBC membrane, inducing partial lysis of the RBC membrane and thereby increasing OF. Thus, the proportion of various fatty acids and particularly that of arachidonic acid is thought to be an important causes of the differences in OF response between the rat, guinea pig and sheep RBCs.
Figure 4:Schematic representation illustrating the distribution of monocarboxylic acids and dicarboxylic acids in the RBC membranes in the rat, guinea pig and sheep. Monocarboxylic acids with a high partition coefficient penetrate deeply into phospholipid layer and have a surfactant-like effect on the rat RBC membrane with loose combination of acyl-chains, but not on the guinea pig or sheep RBC membrane with a rigid combination of acyl-chains. Monocarboxylic acids with a low partition coefficient locate close to water-lipid interface and do not have a surfactant effect. Dicarboxylic acids also locate close to the water-lipid interface and fill the space between the phospholipid heads and acyl-chain roots in the RBC membrane in both species. We previously proposed that the effect of dicarboxylic acids, including isomers of phthalic acids, on the RBC membrane can be regarded as a “wedge-like effect” [11,13,14].
Contrary to the monocarboxylic acids, the application of dicarboxylic acids decreased or tended to decrease OF in the rat, guinea pig and sheep RBCs. These shared OF responses induced in the RBCs from different species could not be explained by the differences in fatty acids composition in the RBC membranes. The partition coefficients of the dicarboxylic acids are lower than those of the corresponding monocarboxylic acids, except for benzoic acid and terephthalic acid. This suggests the moieties of the dicarboxylic acids are more hydrophilic than those of the relating monocarboxylic acids. Thus, the dicarboxylic acids are not able to penetrate far into the hydrophobic lipid region of the RBC membrane from the water-lipid interface (Figure 4). There is a space enclosed by the roots of two acyl-chains and head group of the phospholipids in the lipid-water interface region of the RBC membrane. The physicochemical conditions of this region do not differ greatly in the RBCs of animal species as there are few unsaturated carbon bounds at the roots of acyl-chains bound to the head group of phospholipid compared to the deeper layer in which the acyl-chains are located, Dicarboxylic acids have lower partition coefficients than the corresponding monocarboxylic acids and two hydrophilic carboxylic groups are situated at both ends of their hydrophobic hydrocarbon chain. As two hydrophilic carboxylic groups could not enter deeply into the acyl-chain layer, these elements could be positioned at the interface of phospholipid layer directed toward the water interface. The hydrophobic hydrocarbon chain is thought to enter the region in which the roots of the acyl-chains are combined the phospholipid heads thereby changing its conformation to form a rigid U- or V-shaped structure (Figure 4). We have already proposed that the stabilizing effect of dicarboxylic acids on the RBC membrane and subsequent increase in OF values against osmotic pressure be referred to as a “wedge-like effect” [11,13,14]. In this situation, the hydrophilic hydrocarbons of dicarboxylic acids could not penetrate far into the hydrophobic acyl-chain region in the phospholipid layer. Thus, the interaction between the dicarboxylic acids and the RBC membrane did not lead to substance/phospholipid micelle formation and subsequent cell lysis as observed for the monocarboxylic acids.
In this study, dimethyl-propionic and 3,3-dunethyl-n-butyric acids, isomers of n-valeric (C4) and n-caproic acids (C5), respectively, as well as benzoic acid (C6) demonstrated strong OF-decreasing effects in sheep RBCs. As both isomers have 2 methyl groups on the benzoic ring positioned opposite the carboxylic group in their moiety, the cross-sectional area of their moiety is larger than that of the parent compound. Benzoic acid has a larger cross-sectional area than dose a monocarboxylic acid with a straight hydrocarbon chain (C6), thus we may need to expand our proposed idea to include the notion that the “wedge-like effect” is induced by not only dicarboxylic acids, but also by some monocarboxylic acids with specific types of branched hydrocarbon chain. In a previous experiment, the permeability of shortchain monocarboxylic acids with straight (formic, acetic, propionic and n-butyric acids) and branched (isovaleric and 3-methylacetic acids) hydrocarbon chain was evaluated using a dipalmitoylphosphatidylcholine bilayer model [29]. This experiment demonstrated that the permeability coefficient of these compounds exhibited a linear correlation with the minimum cross-sectional area of the compound, but a poor correlation with molecular volume. It is thought that the form of the branched hydrocarbon chain is important for interaction with the acyl-chain of the phospholipids in the cell membrane.
Further experiments using RBCs from other species in which RBC membranes have phospholipid layers with various fatty acids compositions could explain the mechanism underlying the carboxylic acid-induced changes in membrane characteristics, particularly the differences between the effects of monocarboxylic acids and dicarboxylic acids. The ratio and type of acyl-chains interacting with the carboxylic acids in the phospholipid layer also need to be clarified. Another interesting problem is the fatty acids composition of the RBC membranes interacting with carboxylic acids, which should encourage the development of artificial membranes that can respond to or recognize certain chemical structures. These widespread approaches should enable researchers to identify the mechanism by which carboxylic acids change membrane resistance to osmolarity. Furthermore various investigations using different types of carboxylic acids with more complex hydrocarbon structures, such as branched or cyclic hydrocarbons, are needed to clarify in greater detail the interactions between the hydrocarbons in chemicals and phospholipids in the cell membrane.
We would like to appreciate the technical staff of the Ecorin Village Farm (Eniwa, Hokkaido) for sampling blood from sheep and offering these samples to our laboratory.