International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Case Report - (2022)
Background: The SARS-COV-2 global pandemic has been noted to have a differential effect on those exposed depending on risk factors such as age, diabetes, cardiovascular disease and cancer.
Case presentation: We report a case series of 12 confirmed positive patients and 12 presumptive positive patients all of whom had either an entirely asymptomatic or relatively mild clinical course. 2 patients had active cancer, 3 patients were cancer survivors, 1 patient without cancer was 74 years old. All patients were treated early in their disease course with vitamin D loading (50,000 IU daily for 3 days), 60 to 240 mg melatonin, and 2000 mg oral vitamin C. The 6 high risk patients and one 59 year old patient were treated with at least 2 intravenous doses of vitamin C. The 2 patients with active cancer received 75 grams of vitamin C (one daily, the other every other day). All of the high risk patients had a nearly asymptomatic clinical course and were tested after 10 days and all had a RT PCR for COVID-19 that was negative. The 17 remaining patients also had a relatively benign course though 4 patients (2 in their 20s, 2 in their 50s) received this treatment course later in the course of their illness; these were notably the only patients who had more than a mild sore throat or low grade fever.
Conclusion: We report these unexpectedly positive clinical outcomes of COVID-19 patients including patients with different risk factors, under supplemental vitamin C and D and melatonin. These supplements have a favorable safety profile and are already being suggested as potentially disease altering therapies in the ongoing COVID-19 pandemic.
COVID-19; Cancer; Vitamin D; Melatonin; Vitamin C
Since the outbreak of the December 2019 SARS-CoV-2 virus and subsequent pandemic there have been to date millions of people infected and over 2 million deaths as of 2/3/2021 [1]. Typical symptoms of COVID-19 are fever, cough, sore throat and fatigue [2], with some experiencing gastrointestinal distress [3]. These symptoms can progress to pneumonia and ARDS. The virus does infect children and young adults with usually, but not always, a mild disease course. Overall it is estimated that 81% of COVID-19 cases are mild, 14% of cases have severe symptoms and another 5% are critically ill. In the elderly as well as in people with current and past cancer diagnosis the disease course is often more severe. It is estimated that of those infected, around 8% of people in their 50s, 12% of those in their 60s, and 16.6% of those in their 70s would require hospital care [4]. One study noted that having a current malignant tumor placed a patient at a 39% risk of ICU admission, mechanical ventilation or death [5]. Having cancer with recent chemotherapy or surgery conferred a 75% risk [6] while being a cancer survivor also significantly increases risk of severe disease [7].
COVID-19 currently is treated with supportive measures while a search for promising novel or repurposed antiviral agents is underway [8]. Certain supplements have been suggested as promising in COVID-19 mitigation and treatment. Vitamin D has well known roles in cellular and innate immunity and has a beneficial effect on cytokine levels [9]. Use of intravenous vitamin C is being studied with hopeful results in the treatment of critically ill patients though not currently accepted as standard of care [10]. A trial of 15 grams of intravenous vitamin C has started for critically ill COVID-19 patients [11]. Melatonin has been studied in acute lung injury as a therapeutic modality [12]. It has been proposed as a therapy for COVID-19 [13]. Melatonin has precedence for safe ultrahigh dosing (100-300 mg/day) in multiple studies, mainly focused on its use as a cancer treatment [14]. Viscum Album (mistletoe) is a popular anticancer botanical [15] that boosts T cell function [16] and has been found to have antiviral properties [17-19].
Our case series includes 24 patients. 12 were confirmed positive with RT-PCR nasopharyngeal swab testing. 12 were close contacts with known positives and were presumed positive. Initial cases were exposed to COVID-19 at an integrative medicine outpatient clinic where they were either receiving care or working. Subsequent cases were friends and family members in direct contact with the initial cases. Our reported cases contain lower risk children and higher risk cancer patients, cancer survivors, and elderly. 13 out of 24 cases had mild symptoms with most occurring in the younger segment. The two patients with active cancer were receiving intravenous infusions of mistletoe (viscum album) and vitamin C for their potential therapeutic effects in cancer. The patient with lung cancer case 11 in Figure 1 had received radiation therapy and a bronchial stent for a collapsed lung 2 weeks before his arrival at our clinic on day 5. He had a moderate pleural effusion noted on a recent CT scan and consistent with clinical exam. He was using hydroxychloroquine at 200mg twice daily as a repurposed medication in cancer due to its purported inhibition of autophagic flux [20]. He received intravenous vitamin C at 75 grams per infusion prior to or concurrent with his suspected exposure (Figure 1).
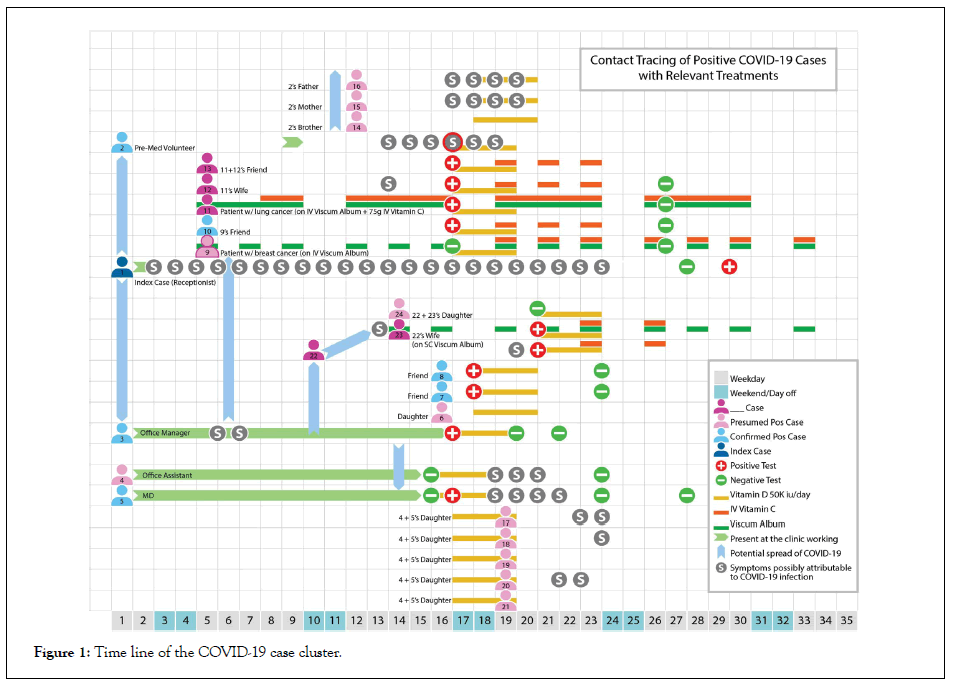
Figure 1: Time line of the COVID-19 case cluster.
The table below lists age, comorbidity, and symptoms of our cases. Only one patient number 22 in Figure 1 had a history of hypertension and was on an ACE inhibitor. No patient was in the either extreme of BMI (Table 1).
| Statistics | ||||||
|---|---|---|---|---|---|---|
| Age | Under 20 | 20-39 | 40-49 | 50-59 | 60-69 | 70-79 |
| #Total (#Presumed) | 7 (7) | 5 (1) | 3 (1) | 4 (1) | 3 (0) | 2 (0) |
| Significant Comorbidities | none | none | 1 with childhood asthma | (1) with active breast cancer | 1 with active lung cancer, 2 cancer survivors | 1 cancer survivor |
| COVID-19 Symptoms | 3 mild (transient chest tightness w/o dyspnea, sore throat) | 2 mild (diarrhea and low grade fever, fatigue) | 2 mild (sore throat and transient chest tightness w/o dyspnea, 2 days backache) | 3 mild (sore throat, headache, fatigue) | 2 mild (low grade fever 1 day, sore throat 1 day) | 1 mild (nausea one day) |
Table 1: Overview of the COVID-19 case cluster.
Cases were tested with nasopharyngeal swab method. The administrator of the test used PPE including an N95 mask, gown, and gloves. A sterile synthetic swab was used with approved viral transport kits and processed by Ipsum Diagnostics, an Atlanta, Georgia based laboratory with FDA Emergency Use Authorization to perform RT-PCR testing for the SARS-CoV-2 virus. Results were typically available after one business day.
Though multiple infection control precautions were put in place already at the clinic (masking staff and patients, social distancing, interval cleaning of surfaces, exclusion of symptomatic staff and patients) this case series demonstrates how easily COVID- 19 is able to spread despite these measures. On day 17 the clinic closed to the public and opened only for patients who had tested positive for COVID-19 or were presumed positive.
Every adult case except the index case was treated with vitamin D dosed at 50,000 IU per day for 3 days. Children were given 20,000 IU/day for 3 days. Oral vitamin C was used at 2000 mg/day divided through the day to prevent loose stools. Melatonin was used in all patients with cancer or respiratory symptoms. Doses from 60 mg to 240 mg per day were used. Intravenous vitamin C was given to 2 patient’s age 50-59, and all patients over 60. In patients without active cancer, vitamin C was given at 15 grams twice over the first week of diagnosis. Both patients with active cancer were receiving more vitamin C as therapies for their cancer- one was getting 75 grams 5 days per week, the other 75 grams 3 days per week. They both were receiving intravenous mistletoe and a third cancer survivor was injecting mistletoe subcutaneously. As the patients felt well they were tested after 7-10 days and found to be negative for COVID-19. Two patients in their 20s (case 1 and 2) and two in their 50s (case 15 and 16) had somewhat more pronounced symptoms. These were all patients who started the vitamin D late or not at all due to a delay in diagnosis. Case 1 was not directly under our care but reported that she had several days of sore throat and sinus congestion. She continued to test positive on day 31. Case 5 had a negative swab 24 hours before a second swab resulted positive. This case had 4 days of mild symptoms and tested negative 8 days later. Viral shedding in patients with COVID-19 is not yet well characterized but one report found that the median viral shedding of hospitalized patients was 15 days with sicker patients tending to shed for longer [21]. It is not possible to accurately estimate the duration of viral shedding of our case cluster. Cases 2, 15 and 16 noticed relief in symptoms after vitamin D supplementation. On three occasions Case 5 reported improvement in his breathing about 2 hours after use of high dose melatonin. Neither patient with active cancer had any symptom at all.
Case 11 had resolution of his pleural effusion confirmed with a chest film day 29 and noted improvement of his breathing despite testing positive for COVID-19. A few cases noted that high dose melatonin caused daytime sleepiness but did not impair daily activities or lower vigilance. No other side effects of the interventions were reported. As the patients were not very ill, bloodwork was not pursued in this patient cluster. No case required hospitalization and all reported they would not have suspected they were infected with COVID-19 if not for having had a positive test (Table 1).
The surprisingly benign clinical course of all of these patients, including those at high risk of severe disease, under treatment with the supplements vitamin C, D and melatonin asks for exploration of such therapies in the use of COVID-19 patients. Vitamin D loading was the therapy most universal. High dose melatonin and intravenous vitamin C was given to all higher risk patients.
All of these therapies are relatively inexpensive, safe if dosed properly, and widely available. Vitamin C is well tolerated with possible side effects of nausea, diarrhea and gastrointestinal side effects at high oral doses over 2 grams in adults [22]. Vitamin C has been found to scavenge free radicals and neutralize inflammatory cytokines- attributes that could hypothetically help against the striking levels of inflammation that occur in COVID-19. Vitamin D has been found in a large meta-analysis to protect against respiratory infections and has a role modulating the immune response- both strengthening innate immunity and decreasing inflammation from immune activity [23]. Melatonin influences immune metabolic pathways to create a less inflammatory situation- potentially countering cytokine storm and having relevance in COVID-19 though more clinical research is needed in this field [24].
Three of the highest risk patients were also using viscum album. Viscum album is European mistletoe extract and is used commonly as an adjunctive cancer therapy mainly by subcutaneous and intravenous routes. It has been found to reduce inflammation through COX 2 specific effects [25,26] Viscum album has a stimulating effect on T cells and immune function in general [27]. All of these therapies have potential impact on the immune system’s interaction with the SARS-CoV-2 virus that could have attenuated the disease course.
Using early case detection to direct Vitamin D supplementation and selective early intravenous Vitamin C and melatonin might have been a safe and effective way to decrease morbidity and mortality from COVID infection in our cases. Use of viscum album in those with cancer may have also given a protective effect. These interventions need to be explored further with trials, especially as they all have a favorable safety profile and are not cost prohibitive.
Informed consent was obtained from all patients or their guardians before publishing any of their details in this report. Patients were given the opportunity to review the published version of this report.
Dr. Hancock owns and operates Humanizing Medicine LLC, an integrative medical clinic that provides intravenous vitamin C and mistletoe as a substantial part of its revenue. Less than 0.5% of yearly revenue is obtained from selling high dose melatonin. Vitamin D is not sold at his clinic. No other conflicts of interest are declared.
No funding was received for the elaboration of this case report.
Not applicable.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Hancock M, Ketterl P, Vinas X, Werthmann P (2022) Case Cluster of RT-PCR COVID-19 Positive Patients with an Unexpected Benign Clinical Course with Vitamin D, Melatonin, Vitamin C, and Viscum Album. Int J Phys Med Rehabil.S17:004.
Received: 22-Apr-2022, Manuscript No. JPMR-22-17116; Editor assigned: 25-Apr-2022, Pre QC No. JPMR-22-17116 (PQ); Reviewed: 13-May-2022, QC No. JPMR-22-17116 ; Revised: 18-May-2022, Manuscript No. JPMR-22-17116 (R); Accepted: 25-May-2022 Published: 25-May-2022 , DOI: 10.35248/2329-9096-22.S17.004
Copyright: © 2022 Hancock M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.