Bipolar Disorder: Open Access
Open Access
ISSN: 2472-1077
ISSN: 2472-1077
Case Report - (2022)Volume 8, Issue 1
Background: There are sporadic case-reports suggesting an association between sodium valproate and neutropenia, though the majority of these are in cases of paediatrics and epilepsy. There are a few cases of psychiatric patients developing neutropenia after newly being started on sodium valproate. We present a patient, previously on long-term sodium valproate therapy, who developed neutropenia after being restarted on sodium valproate.
Case report: A 52-year-old Chinese man with bipolar disorder, previous well on a combination of sodium valproate and risperidone, was re-admitted for a manic relapse after a period of non-compliance. He developed neutropenia that coincided with increases in sodium valproate dose, and only resolved upon discontinuation of sodium valproate, despite continued increases in risperidone dose. Other investigations for neutropenia, including physical examination and blood tests, were unremarkable. He was eventually stabilized on a combination of risperidone and aripiprazole.
Conclusions: This report adds to the growing evidence for sodium valproate-induced neutropenia. Given the frequency of prescription of sodium valproate in bipolar disorder, and the potential serious consequences of neutropenia, this is an area that merits large-scale research.
Sodium valproate; Valproate; Neutropenia; Agranulocytosis; Induced
Full Blood Count (FBC); White Cell Count (WCC); Granulocyte Colony Stimulating Factor (GCSF); United States Food and Drugs Administration (FDA)
Sodium valproate is a commonly used medication for both seizure-control in patients with epilepsy as well as mood stabilization in patients suffering from bipolar disorder. It can cause various hematological dyscrasias, the most common of which is thrombocytopenia [1].
From our literature review, we found several case-reports of sodium valproate-related neutropenia (a reduction in absolute neutrophil count to under 1,500/μL) [2-7]. Most of these case reports were in pediatrics and epilepsy. Few reported on sodium valproate-related neutropenia in bipolar disorder [8,9]. We present the case of a middle-aged gentleman, previously stable for many years on sodium valproate, who developed neutropenia after being restarted on it following a period of non-compliance to treatment. We also discuss the possible mechanisms and management of sodium valproate-induced neutropenia.
We present the case of a 52-year-old Chinese man with a known history of bipolar disorder, who was maintained on sodium valproate 1500 mg and risperidone 1 mg nightly for several years without requiring admissions. He was brought into the emergency department for a manic relapse, following a period of non-compliance. Sodium valproate was restarted at 500 mg nightly and he was admitted for further treatment. Subsequently, it was increased to 1000 mg nightly and risperidone 0.5 mg nightly was added. Routine blood tests were done the next morning, including fasting glucose and lipids, liver panel, renal panel, thyroid function tests and full blood count (FBC), which showed neutropenia (White cell count (WCC) 2,900/μL, Neutrophils 1,020/μL). Other cell lines were unaffected (Hemoglobin 13.5 g/dL, Platelets 247/μL), and the other blood tests were unremarkable. The patient did not exhibit any signs of ongoing infection (such as dental caries), hematological malignancies (such as hepatosplenomegaly) or nutritional deficiencies (such as peripheral neuropathy) upon physical examination. He had no significant past medical or family history. As the neutropenia was not severe (<1,000/μL) the decision was made to reduce the sodium valproate dose back to 500 mg nightly. FBC and serum valproate levels were taken two days later. The valproate level was 56 mg/L and the neutropenia resolved (WCC 4,800/μL, Neutrophils 2,450/μL). Further history revealed that he had been taking clarithromycin for an upper respiratory tract infection prior to admission. Considering that clarithromycin has also been linked to neutropenia [10], and that he was relatively well on sodium valproate before, the decision was made to increase the dose back to 1000 mg nightly with frequent FBC monitoring, as he remained symptomatic for mania. However, a subsequent FBC showed neutropenia again (WCC 3,200/μL, Neutrophils 1,210/ μL), and the dose was reduced back to 500 mg. The neutropenia was sustained, despite the reduction in dose, two days later (WCC 3,200/μL, Neutrophils 1,070/μL), and sodium valproate was discontinued. Other investigations for neutropenia, including B12 and Folate levels and a peripheral blood film, were unremarkable. Repeat liver and renal function tests were also unremarkable. Repeat FBCs on days 17 (WCC 3,800/μL, Neutrophils 1,500/μL), 19 (WCC 4,100/μL, Neutrophils 1,600/μL) and 27 (WCC 4,100/μL, Neutrophils 1,800/μL) postadmission showed recovery of WCC and neutrophil counts. Table 1 and Figure 1 below illustrate the relationship between the patient’s daily sodium valproate doses and his measured neutrophil counts.
| Day | Neutrophil count/µL | Daily Sodium Valproate dose (mg) |
|---|---|---|
| 1 | 500 | |
| 2 | 1000 | |
| 3 | 1020 | 1000 |
| 4 | 500 | |
| 5 | 2450 | 500 |
| 6 | 500 | |
| 7 | 500 | |
| 8 | 500 | |
| 9 | 500 | |
| 10 | 1000 | |
| 11 | 1210 | 1000 |
| 12 | 500 | |
| 13 | 1070 | 0 |
| 14 | 0 | |
| 15 | 0 | |
| 16 | 0 | |
| 17 | 1500 | 0 |
| 19 | 1600 | 0 |
| 27 | 1800 | 0 |
Table 1: Neutrophil counts and daily Sodium Valproate doses during the course of admission.
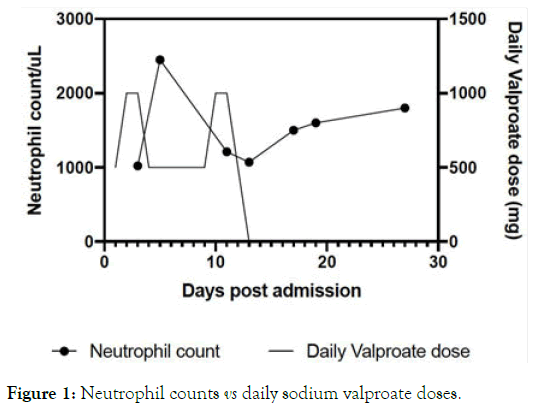
Figure 1. Neutrophil counts vs daily sodium valproate doses.
Although there are case reports suggesting risperidone as a potential cause of neutropenia [11,12], this seems less likely as the neutropenia resolved despite the progressive increase in risperidone dose, similar to a previously reported case [9]. Sodium valproate seems to be the most likely cause of the patient’s neutropenia due to the temporal relationship between its administration and the neutropenia, as illustrated in Figure 1. It was decided not to restart sodium valproate. An adverse drug event report was filed in the local Critical Medical Information System to alert the Health Sciences Authority and other healthcare providers of this potential adverse reaction. The patient was eventually stabilized on a combination of 6 mg of risperidone and 5 mg of aripiprazole. As he remained afebrile, non-toxic and maintained neutrophil counts above 1000/μL, Granulocyte Colony Stimulating Factor (G-CSF) and broad-spectrum antibiotics were not indicated.
In our literature review, we did not find any guidelines specific to the management of sodium valproate-related neutropenia in bipolar disorder. The mechanism behind sodium valproateinduced neutropenia is poorly understood. The first study to report this phenomenon was unable to detect antibodies against neutrophils in the patient ’ s serum [3]. Direct bone-marrow suppression has been proposed as a mechanism, with one casereport finding a dose-dependent relationship between sodium valproate dosage and bone-marrow suppression [13,14]. However, findings from bone-marrow biopsies in subsequent case reports are contrasting, with one report finding arrest of the myeloid maturation process in a 75-year-old patient ’ s bone marrow [7], and another finding no abnormalities in a 2-yearold’s bone-marrow [6]. In our case, we were not able to ascertain a relationship between serum valproate levels and neutropenia, as only a single valproate level was checked on day 5 postadmission. The relationship between the two is unclear, and studies have found that neutropenia may occur even with subtherapeutic serum valproate levels [15].
The main complication of neutropenia is secondary infection. Though patients can be asymptomatic and afebrile, it is important to monitor for signs of infection such as dental caries or abscesses, as this can be a marker of poor bone-marrow reserve and increases the risk of life-threatening sepsis. A fever should always be considered a sign of infection, as these patients may lack localizing symptoms [16]. In at least two of these cases, patients developed serious complications and co-morbidities that required intensive treatment and administration of G-CSF for recovery of neutrophil levels [5,7]. G-CSF is generally used prophylactically for the reduction in risk of infection in patients with lympho/myeloproliferative disorders, receiving hematopoietic stem-cell/bone-marrow transplantation or undergoing chemotherapy, with a greater than 20% risk of febrile neutropenia [17,18]. It is United States Food and Drug Administration (FDA) approved for the above indications and severe chronic neutropenia [18,19]. There are currently no guidelines for the use of G-CSF in drug-induced neutropenia, though it is suggested that it be avoided except in cases of recurrent infections, regardless of the severity of the neutropenia [16]. Some studies have suggested a neutrophil count of less than 100/μL as an indication for G-CSF in drug-induced neutropenia [20,21].
The safest approach to managing any severe drug-induced neutropenia would be to discontinue the most probable culprit drug [22]; the neutropenia is expected to resolve within one to four weeks after [5-7]. There are currently no guidelines to address whether the suspected drug should be withheld permanently or subsequently re-challenged. A recent systemic review suggested that for cases with mild neutropenia, continuation of the implicated drug could be considered, with strict monitoring. The author also suggests for temporary cessation of the drug and reinstatement once neutrophil count normalises for cases with moderate neutropenia, and that alternative agents should be used for severe cases [22]. Local guidelines also recommend annual checks of FBCs for stable patients on sodium valproate [23,24].
In conclusion, we found a significant lack of literature on sodium valproate-induced neutropenia. There are patients with bipolar disorder who may greatly benefit from the long-term use of sodium valproate, and it is one of the first line medications for the acute and maintenance treatment of bipolar disorder. This is hence an area that requires significant attention, given the potential severity of neutropenia and the relatively high frequency of sodium valproate prescription for bipolar disorder, and will require research utilizing large national registries and databases.
Consent for publication
The subject of this case-report has consented to the publication of this report.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Arvind Rajagopalan conceived the original idea for the manuscript. Arvind Rajagopalan, Shuli Lim, Christopher Yi Wen Chan, Yee Ming Mok and Nisha Chandwani contributed to the writing and proofreading of the final manuscript. The final manuscript was discussed and approved by all authors.
Citation: Arvind R, Shuli L, Yi Wen CC, Yee Ming M, Nisha C (2020) Case Report: Sodium Valproate-Induced Neutropenia in a Patient with Bipolar Disorder. Bipolar Disord 8:166. doi:10.35248/2472-1077.22.8.166
Received: 03-Jan-2022, Manuscript No. JBD-22-15255; Editor assigned: 06-Jan-2022, Pre QC No. JBD-22-15255; Reviewed: 17-Jan-2022, QC No. JBD-22-15255; Revised: 24-Jan-2022, Manuscript No. JBD-22-15255; Published: 31-Jan-2022 , DOI: 10.35248/2472-1077.22.8.166
Copyright: © 2022 Arvind R, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited