Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2024)Volume 15, Issue 6
Objective: Aimed to explore the protective effects of cassia polysaccharides on myopia by examining their influence on Adult Retinal Pigment Epithelium-19 (ARPE-19) cells with reduced Paired Box Protein (PAX6) expression.
Methods: ARPE-19 cell line with diminished PAX6 expression was established using a lentiviral approach and addition of XAV-939, an inhibitor of the Wnt/β-catenin. We assessed the expression of genes and proteins involved in the Wnt family member 2 (Wnt/β-catenin, scleral remodeling and cell cycle regulation following treatment with cassia polysaccharides. Gene and protein expression were quantified using Reverse Transcription Polymerase Chain Reaction (RT-PCR) and western blot analyses, respectively. Additionally, the migratory capabilities of these cells were evaluated using a scratch assay.
Results: Optimal transduction was achieved with a Multiplicity of Infection (MOI) of 20, successfully generating a stable ARPE-19 cell line with low PAX6 expression. Cassia polysaccharides did not significantly alter the expression of Wnt2 compared to control groups. Similarly, when treated with XAV-939, β-catenin levels were modified in the PAX6-shRNA and XAV-939 but remained unchanged in the cassia polysaccharide. Scleral remodeling markers, including Matrix Metalloproteinase-2 (MMP-2) and Transforming Growth Factor-β (TGF-β), were elevated and Collagen Type 1 Alpha 1 (COL1A1) was decreased in the PAX6-shRNA, with no significant changes observed in the cassia polysaccharide. Cell cycle analysis indicated reduced Cyclin-Dependent Kinase 1 (CDK1) and Proliferating Cell Nuclear Antigen (PCNA) levels in the PAX6-shRNA: Paired box protein 6-short hairpin ribonucleic acid (PAX6-shRNA), with cassia polysaccharides showing no significant effect. Scratch assay results demonstrated slower wound healing in the PAX6-shRNA compared to controls over 72 h with no significant differences observed in the cassia polysaccharide.
Conclusion: Cassia polysaccharides may mitigate ARPE-19 cell damage induced by low PAX6 expression through modulation of the Wnt/β-catenin, potentially slowing the progression of myopia and offering a protective effect on vision.
Cassia polysaccharide; PAX6 gene; Wnt/β-catenin; Myopia
Myopia is one of the most common eye diseases and is a common concern worldwide [1,2]. The prevalence of myopia among high school students, especially in some parts of East and Southeast Asia, reaches 80%-90% [3]. Myopia imposes a public health burden of refractive error correction on patients and society and affects quality of life [4]. In addition to the earlier myopia occurs, the more likely it is to progress to high myopia, increasing the risk of potential vision pathologies such as macular degeneration [5,6]. Therefore, it is important to prevent the development of myopia.
The Wnt/β-catenin signaling pathway is a type of signaling pathway that is highly conserved during species evolution and plays an important role in animal embryonic development and adult tissue homeostasis and compared to other tissues around the eye, the Wnt/β-catenin signaling pathway is highly expressed in corneal tissues [7,8]. The PAX6 gene is one of the major regulators of ocular vision and plays an important regulatory role in vertebrate eye development, especially in the formation and development of the lens and corneal epithelium [9,10]. PAX6 consists of 16 exons and three promoter regions (P0, P1 and Pα), resulting in three isoforms of the PAX6 protein, i.e., classical PAX6, PAX6 (5a) and PAX6 (ΔPD) [11]. In addition, in vertebrates, the PAX6-encoded product affects the expression of Transforming Growth Factor-β2 (TGF-β2) and Follistatin (Fst), which also regulate the function of PAX6 through positive and negative feedback [12].
There are three highly homodimeric forms of TGF-β (TGF-β1, 2 and 3), which mediate cellular effects through the same receptor complex and affect important physiological processes such as cell growth, differentiation and immunity [13]. Among them, TGF-β1, which has the highest level of expression in human tissues, plays an important role in scleral remodeling, myofibroblast transdifferentiation and the formation of high myopia by regulating the expression of collagen in the Extracellular Matrix (ECM), especially COL1A1 [13,14]. Scleral remodeling is a dynamic process involving continuous synthesis and degradation of the ECM and MMP-2, a member of the endopeptidase family, has the ability to degrade ECM components and participate in signal transduction [15,16]. It has been shown that MMP-2 overexpression can affect scleral tissue remodeling, which in turn leads to the onset and development of pathological myopia [17]. And it was experimentally confirmed that knocking down MMP-2 in a mouse deprivation myopia model significantly increased the accumulation of COL1A1 in the sclera during myopia [18].
Retinal Pigment Epithelium (RPE) cells are pigment cells that develop and differentiate from "optic nerve vesicles" and play an important role in maintaining retinal homeostasis and integrity [19]. Oxidative stress accelerates the senescence of RPE cells, resulting in the loss of their hexagonal structure and a corresponding weakening of their barrier function, leading to altered retinal function [20,21].
Cassia seeds, originally documented in the ancient Chinese medical text "Shennong Ben Cao Jing" are known for their rich bioactive constituents including anthraquinones, fatty acids, naphthalene, pyrrolidone and polysaccharides. These components confer a range of physiological benefits such as antioxidant, anti-aging, blood pressure and lipid regulation, antibacterial, anti-inflammatory effects and obesity inhibition. Specifically, cassia polysaccharides, a primary active component, exhibit antioxidant, anti-inflammatory and immunomodulatory properties. These properties are essential in preventing and mitigating hereditary ocular diseases like retinitis pigmentosa. Historically, as noted in the "Divine Husbandman's Classic of the Materia Medica" Cassia seeds have been primarily used to treat various eye-related ailments including glaucoma, redness, swelling, pain, cataracts and tearing. Cassia polysaccharides effectively neutralize reactive oxygen species such as O2-, -OH and HO2 and reduce Malondialdehyde (MDA) production, thus enhancing the body’s antioxidant capability. This action helps prevent eye diseases caused by oxidative damage and slows their progression.
Given this background, our research aims to develop a cellular model of myopia to explore the protective effects of cassia polysaccharides on human retinal pigment epithelial cells. This study will particularly focus on the modulation of the Wnt/β-catenin signaling pathway and assess the expression of related factors, providing insights for the prevention and management of myopia.
Materials and reagents
ARPE-19 cells were purchased from National Collection of Authenticated Cell Cultures, Chinese Academy of Sciences; DMEM/F12K medium was purchased from Gibco; cassia polymorpha was purchased from ichuan Weikeqi Biological Technology Company Limited Sichuan, China; PAX6 antibody and sheep anti-rabbit antibody were purchased from Proteintech Group, Incorporated Wuhan, China; Wnt2 antibody, β-catenin antibody, COL1A1 antibody, MMP-2 antibody, TGF-β antibody, cyclin D1 antibody, PCNA antibody were purchased from Wanleibio Co., Liaoning, China; β-actin antibody and goat anti-mouse antibody were purchased from Beyotime Biotechnology Shanghai, China.
All primer sequences were synthesized and acquired by Sangon Biotech Shanghai, China (Table 1), lentivirus was packaged and acquired by OBiO Technology Shanghai, China; XAV-939 was purchased from Aladdin Biochemical Technology Company Shanghai, China.
Cell culture
ARPE-19 cells were cultured in 37°C, 5% carbon dioxide (CO2) incubator, using Dulbecco's Modified Eagle Medium/Ham's F-12K (DMEM/F12K) medium containing 10% Fetal Bovine Serum (FBS) and 1% penicillin-streptomycin cell density of 90% or more can be sub-cultured, digested with 0.05% trypsin for 3 min and then terminated by microscopic observation of the cells were detached and rounded, centrifuged at 2500 rpm for 5 min, cultured at 1:3. Cell densities of 80%-90% were achieved around 5 days. The cryopreservation solution for freezing was 70% fetal bovine serum, 20% complete medium and 10% Dimethyl Sulfoxide (DMSO) and the program was cooled to -80°C and transferred to liquid nitrogen the following day.
Constructing cellular model of myopia
ARPE-19 cells were inoculated in six-well plates at a density of 1 × 106 cells/ml and ARPE-19 cells were infected with MOI (number of viruses infected per live cell)=0, 2.5, 5, 10, 20, 40 and equal volume of pro-transfection reagent was added and the cell infection was observed under a fluorescence microscope after incubation for 24 h with serum-free and antibiotic-free medium. After infecting the cells with appropriate MOI, ARPE-19 cells were cultured for two weeks using medium containing 2 μg/L puromycin to remove the lentivirus-uninfected cells and a stable low-expression ARPE-19 cell line of PAX6 gene was constructed and the expression level of PAX6 was detected by RT-PCR and western blot assay.
Western blot assay
Cells were washed with pre-cooled Phosphate-Buffered Saline (PBS), collected with a cell scraper, added with lysate containing 1/100 of trypsin inhibitor and 1/50 of phosphoproteinase inhibitor and lysed for 30 min on ice and then centrifuged at 4°C for 15 min at 12,000 rpm. The supernatant was collected and protein concentration was determined by Bicinchoninic Acid (BCA) method. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) gels were prepared and electrophoresed at 80 V to the point of separation and then changed to 120 V until the end of electrophoresis. After trarsmembraned with 200 mA current for 1 h-1.5 h, the Polyvinylidene Fluoride (PVDF) membrane was placed in 5% sealing solution and blocking for 2 h. Subsequently, diluted antibody was added and incubated at 4°C overnight, the secondary antibody was incubated on the next day and protein bands were detected by Enhanced Chemiluminescence (ECL) chemiluminescence.
Reverse transcription polymerase chain reaction assay
Cells were washed with pre-cooled PBS, total Ribonucleic Acid (RNA) was extracted by the TRIzol and reverse transcription was performed with the complementary Deoxyribonucleic Acid (cDNA) first strand synthesis kit (RNase H-), with reaction was carried out at 42°C for 60 min and at 80°C for 10 min to inactivate the reverse transcriptase and terminate the reverse transcription reaction.
The reaction system for RT-PCR was 10 μl SYBR Green (SG), 0.4 μl each of forward primer and reverse primer, 1 μl of cDNA and double-distilled Water (ddH2O) to make up to 20 μl. Reaction conditions were pre-denaturation 95°C for 30 s, 40 cycles of reaction 95°C for 10 s and 60°C for 30 s and a dissolution curve 60°C for 60 s.
Scratch assay
ARPE-19 cells were inoculated in six-well plates at a density of 1 × 106 cells/ml and when the cells were evenly spread all over the six-well plates, the serum-free antibiotic-free medium was replaced and scratched and the migration status of the cells was observed every 24 h and the cells were observed for 72 h consecutively, the assay was repeated three times independently.
Statistical analysis
Data for each group are expressed as mean ± Standard Deviation (SD) and the significance of differences in means between groups was determined using GraphPad Prism's Student's t-test, with p<0.05 being considered statistically significant and repeated at least three times for each group.
Establishing a myopic cell line model with stable low expression of PAX6
After infecting ARPE-19 cells with 0, 2.5, 5, 10, 20, 40 of MOI respectively for 48 h, results showed that the best infection efficiency was achieved when MOI was 20 (Figure 1). Which down-regulated PAX6 gene expression was detected, down-regulated PAX6 protein expression was detected in ARPE-19 cells even at 72 h (Figures 2 and 3). The results of ARPE-19 cell line with stable low expression of PAX6 means a myopic cell line model was successfully established.
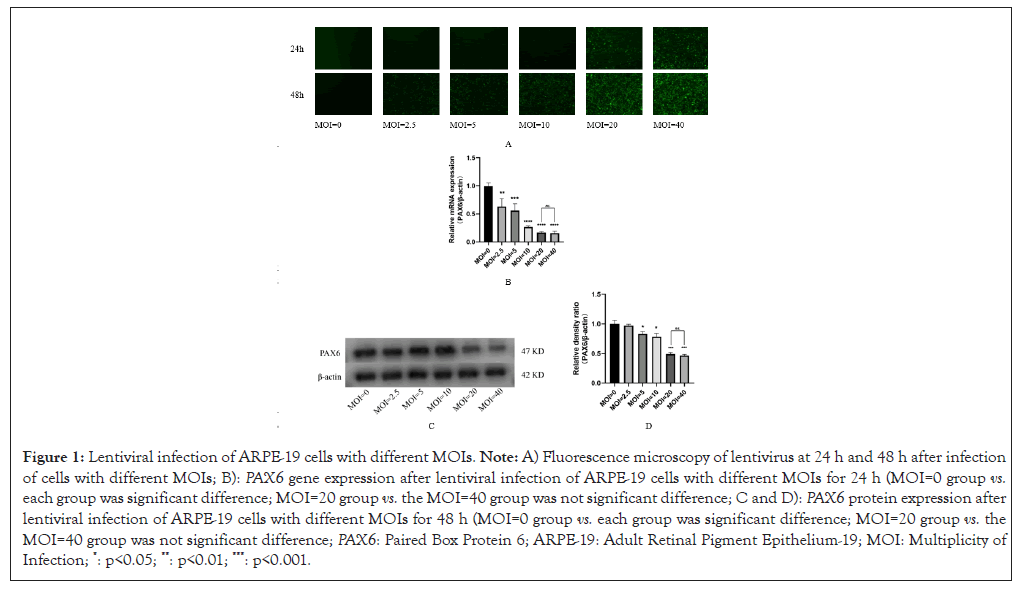
Figure 1: Lentiviral infection of ARPE-19 cells with different MOIs. Note: A) Fluorescence microscopy of lentivirus at 24 h and 48 h after infection of cells with different MOIs; B): PAX6 gene expression after lentiviral infection of ARPE-19 cells with different MOIs for 24 h (MOI=0 group vs. each group was significant difference; MOI=20 group vs. the MOI=40 group was not significant difference; C and D): PAX6 protein expression after lentiviral infection of ARPE-19 cells with different MOIs for 48 h (MOI=0 group vs. each group was significant difference; MOI=20 group vs. the MOI=40 group was not significant difference; PAX6: Paired Box Protein 6; ARPE-19: Adult Retinal Pigment Epithelium-19; MOI: Multiplicity of Infection; *: p<0.05; **: p<0.01; ***: p<0.001.
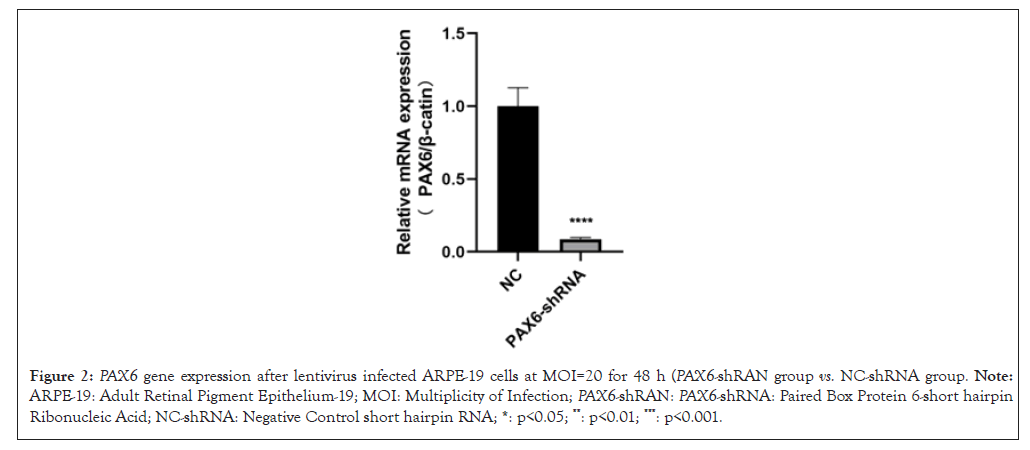
Figure 2: PAX6 gene expression after lentivirus infected ARPE-19 cells at MOI=20 for 48 h (PAX6-shRAN group vs. NC-shRNA group. Note: ARPE-19: Adult Retinal Pigment Epithelium-19; MOI: Multiplicity of Infection; PAX6-shRAN: PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid; NC-shRNA: Negative Control short hairpin RNA; *: p<0.05; **: p<0.01; ***: p<0.001.
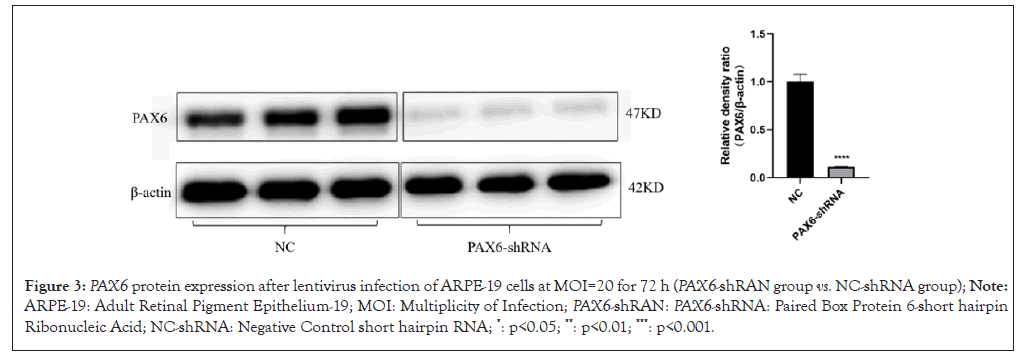
Figure 3: PAX6 protein expression after lentivirus infection of ARPE-19 cells at MOI=20 for 72 h (PAX6-shRAN group vs. NC-shRNA group); Note: ARPE-19: Adult Retinal Pigment Epithelium-19; MOI: Multiplicity of Infection; PAX6-shRAN: PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid; NC-shRNA: Negative Control short hairpin RNA; *: p<0.05; **: p<0.01; ***: p<0.001.
Cassia polysaccharides can modulate the effect of low PAX6 expression on the Wnt/β-catenin pathway
The results of western blot and RT-PCR experiments showed that the expression of Wnt2 genes and proteins in both the PAX6-shRNA group and the XAV-939 group was increased compared with the blank group, but the expression of β-catenin in the XAV-939 group was down-regulated compared with the blank group (Figure 4). It suggests that the reduction of PAX6 gene expression controls the activation of the Wnt/β-catenin signaling pathway by affecting the expression of upstream Wnt, rather than directly regulating downstream factors such as β-catenin. However, the differences in Wnt2 and β-catenin expression in the cassia polysaccharide group were not statistically significant compared with the blank group (Figure 4), suggesting that cassia polysaccharide can negatively regulate the overexpression of Wnt2 induced by the low expression of PAX6 gene.
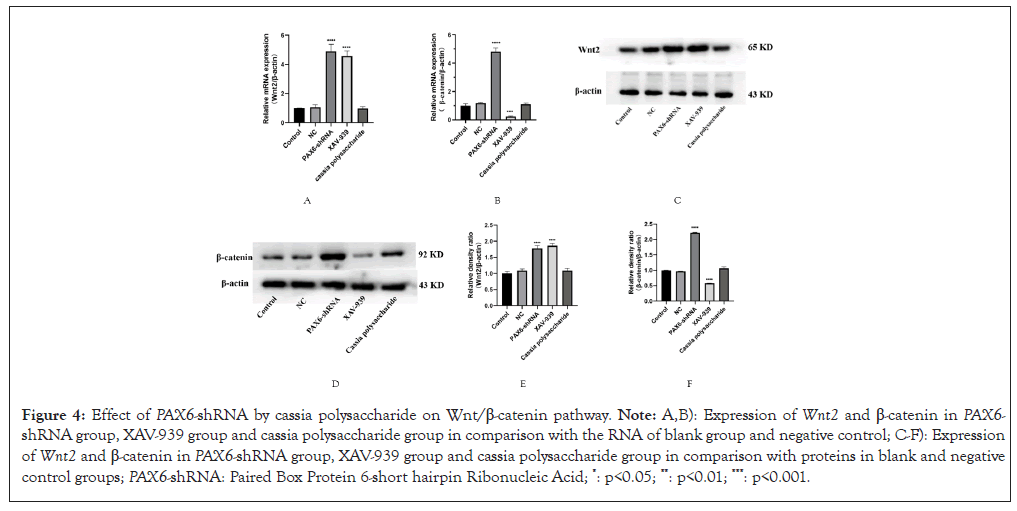
Figure 4: Effect of PAX6-shRNA by cassia polysaccharide on Wnt/β-catenin pathway. Note: A,B): Expression of Wnt2 and β-catenin in PAX6-shRNA group, XAV-939 group and cassia polysaccharide group in comparison with the RNA of blank group and negative control; C-F): Expression of Wnt2 and β-catenin in PAX6-shRNA group, XAV-939 group and cassia polysaccharide group in comparison with proteins in blank and negative control groups; PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid; *: p<0.05; **: p<0.01; ***: p<0.001.
Cassia polysaccharide modulates the effect of PAX6-shRNA on scleral remodeling
Scleral remodeling also plays an important role in the development of myopia and detection of low PAX6 gene expression is particularly important for the expression of scleral remodeling-related proteins and RNAs. We found that the expression of COL1A1 was decreased and MMP-2 and TGF-β were elevated in the PAX6-shRNA group compared with the negative control group, while the expression of COL1A1, MMP-2 and TGF-β in the cassia polysaccharide group was in the opposite direction (Figure 5), which suggests that cassia polysaccharide has a protective effect on ARPE-19 cells caused by low expression of PAX6 gene had a certain protective effect on scleral remodeling.
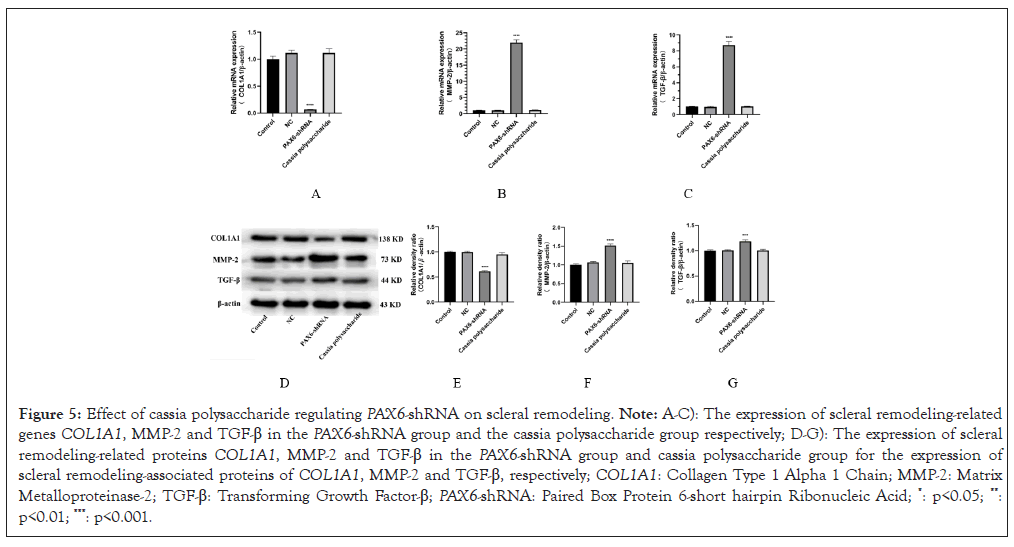
Figure 5: Effect of cassia polysaccharide regulating PAX6-shRNA on scleral remodeling. Note: A-C): The expression of scleral remodeling-related genes COL1A1, MMP-2 and TGF-β in the PAX6-shRNA group and the cassia polysaccharide group respectively; D-G): The expression of scleral remodeling-related proteins COL1A1, MMP-2 and TGF-β in the PAX6-shRNA group and cassia polysaccharide group for the expression of scleral remodeling-associated proteins of COL1A1, MMP-2 and TGF-β, respectively; COL1A1: Collagen Type 1 Alpha 1 Chain; MMP-2: Matrix Metalloproteinase-2; TGF-β: Transforming Growth Factor-β; PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid; *: p<0.05; **: p<0.01; ***: p<0.001.
Regulation of PAX6-shRAN-influenced cell cycle by cassia polysaccharides
Low PAX6 expression affects the cell cycle, resulting in slower growth of ARPE-19 cells with low PAX6 expression under the same culture conditions, whereas the cell growth status of the cassia polysaccharide group did not differ much from that of the negative control group. Subsequently, we detected the expression of cell cycle-related genes and proteins using western bolt and RT-PCR and found that the expression of CDK1 and PCNA in the PAX6-shRNA group was reduced compared with that of the negative control group, whereas the expression of CDK1 and PCNA was normal in the cassia polysaccharide group (Figure 6). It indicated that cassia polysaccharide could regulate the cell cycle hysteresis caused by low PAX6 expression and protect the normal function of ARPE-19 cells.
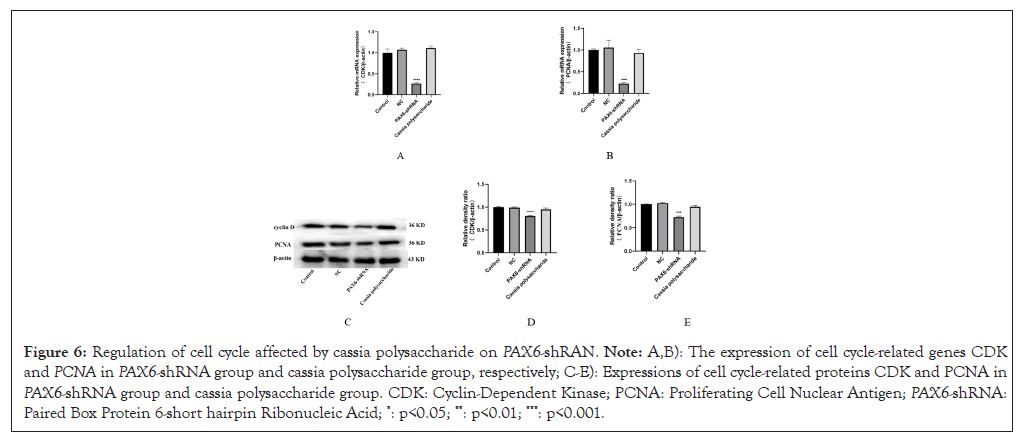
Figure 6: Regulation of cell cycle affected by cassia polysaccharide on PAX6-shRAN. Note: A,B): The expression of cell cycle-related genes CDKand PCNA in PAX6-shRNA group and cassia polysaccharide group, respectively; C-E): Expressions of cell cycle-related proteins CDK and PCNA in PAX6-shRNA group and cassia polysaccharide group. CDK: Cyclin-Dependent Kinase; PCNA: Proliferating Cell Nuclear Antigen; PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid; *: p<0.05; **: p<0.01; ***: p<0.001.
Scratch assay to detect the rate of cell migration
Abnormal expression of cell cycle-related genes and proteins also suggested changes in cell migration function, so we performed scratch experiments on ARPE-19 cells from different treatment groups and observed them continuously for 72 h (Figure 7). From the results, it can be seen that the low expression of PAX6 leads to the decrease of cell migration, while cassia polysaccharide can increase the migration of ARPE-19 cells after the low expression of PAX6 gene to a certain extent and the scratch wound healing is faster than that of the PAX6-shRNA group. The difference in cell migration between cassia polysaccharide group and blank group and negative control group was not significant, indicating that cassia polysaccharide could protect the migration function of ARPE-19 cells after low expression of PAX6.
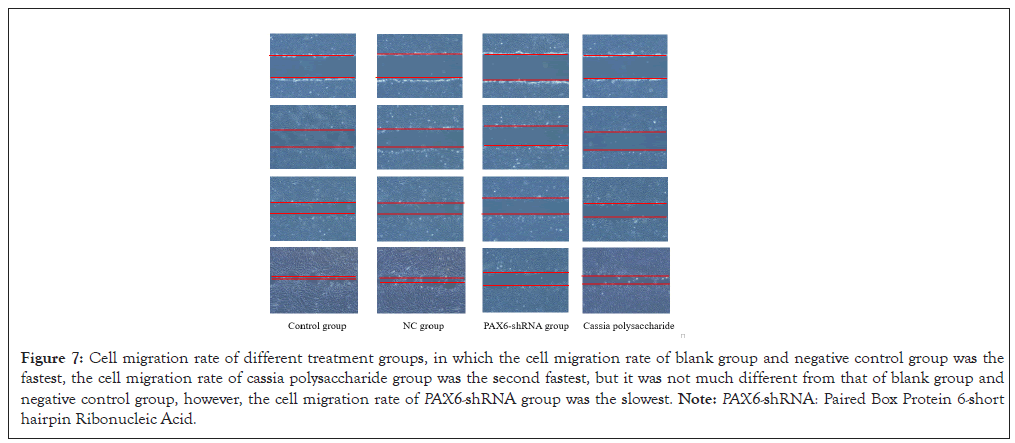
Figure 7: Cell migration rate of different treatment groups, in which the cell migration rate of blank group and negative control group was the fastest, the cell migration rate of cassia polysaccharide group was the second fastest, but it was not much different from that of blank group and negative control group, however, the cell migration rate of PAX6-shRNA group was the slowest. Note: PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid.
Cassia polysaccharides can modulate the effect of low PAX6 expression on the PI3K/AKT-P27 pathway
After testing the effect of cassia seed polysaccharide-regulated PAX6 low expression on the Wnt/β-catenin pathway and cell cycle, we also tested the expression of factors related to the PI3K/AKT-P27 pathway. We found that PAX6 low expression inhibited the PI3K/AKT-P27 pathway and the expression of genes and proteins of PI3K and AKT in the PAX6-shRNA group was lower than that in the control group and the cassia seed polysaccharide group, whereas the expression of P27 in the PAX6-shRNA group was elevated compared with that in the control group and the cassia seed polysaccharide group (Figure 8).
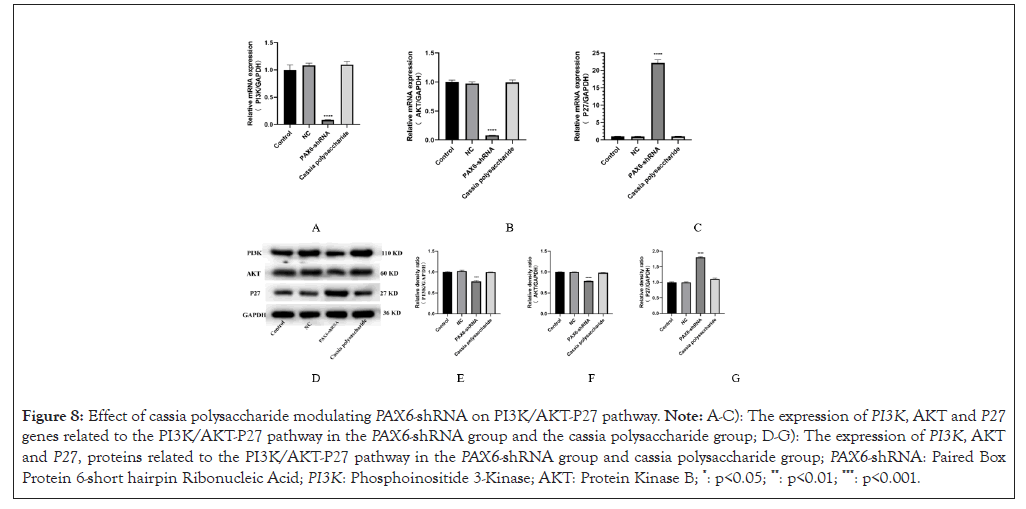
Figure 8: Effect of cassia polysaccharide modulating PAX6-shRNA on PI3K/AKT-P27 pathway. Note: A-C): The expression of PI3K, AKT and P27 genes related to the PI3K/AKT-P27 pathway in the PAX6-shRNA group and the cassia polysaccharide group; D-G): The expression of PI3K, AKT and P27, proteins related to the PI3K/AKT-P27 pathway in the PAX6-shRNA group and cassia polysaccharide group; PAX6-shRNA: Paired Box Protein 6-short hairpin Ribonucleic Acid; PI3K: Phosphoinositide 3-Kinase; AKT: Protein Kinase B; *: p<0.05; **: p<0.01; ***: p<0.001.
The PAX6 gene is critical for ocular development and has been associated with the development of several ocular diseases [22,23]. To date, the association of PAX6 gene polymorphisms with myopia and refractive error remains controversial. PAX6 was not found to be associated with myopia in caucasians [24], whereas two Single Nucleotide Polymorphisms (SNPs) in PAX6-rs3026390 and rs3026393 were found to be significantly associated with high myopia in Chinese [25,26]. PAX6 polymorphisms have also been associated with high myopia in Chinese adults, but the association of PAX6 with high myopia has not been reported in Chinese children [27,28].
In this study, we used lentiviral infection of ARPE-19 cells to successfully establish a myopic cell model with stable low expression of PAX6. We found that the expression of Wnt2 and β-catenin proteins in ARPE-19 cells was elevated after the low expression of PAX6, whereas the expression of Wnt2 and β-catenin proteins basically restored to normal after the addition of cassia polysaccharide. In order to verify the relationship between PAX6 and cassia polysaccharide and the Wnt/β-catenin signaling pathway, we subsequently added XAV-939, a blocker of the Wnt/β-catenin signaling pathway and found that Wnt2 expression was up-regulated, whereas downstream β-catenin expression was reduced. This demonstrated that PAX6 and cassia polysaccharides affect the Wnt/β-catenin signaling pathway by regulating Wnt protein expression.
A major cause of myopia is refractive error due to growth of the eye axis, which is closely related to the remodeling of the scleral extracellular matrix. Scleral remodeling leads to changes in the axial length of the eye and refractive status, which are important features of myopia. TGF-β is expressed in the sclera, retina and choroid and is involved in the formation of Form Deprivation Myopia (FDM) [29-31], which also affects scleral remodeling and the formation of high myopia [32]. Loss of COL1A1 function leads to systemic diseases such as osteogenesis imperfecta, scleral thinning and myopia. Another study has shown that microR-328 can reduce PAX6 expression levels by binding to the 3'-Untranslated Region (UTR) region of PAX6 causing an increase in MMP-2 expression, which leads to enhanced extracellular matrix degradation and scleral thinning and lengthening of the ocular axis, thus affecting the progression of myopia [33]. Upregulation of MMP-2 leads to thinning of the scleral collagen framework and changes in extracellular matrix composition, inducing the development of myopia. These are consistent with the results of the present study.
In addition, we found that the ARPE-19 cells in the PAX6-shRNA group grew slower than the other groups under the same conditions during the culture of ARPE-19 cells, with the question, we also examined the expression of cell cycle-related genes and proteins in different groups and found that the expression of cell cycle-related genes and proteins cyclin D1 and PCNA were reduced in ARPE-19 cells in PAX6-shRNA group. Then, we tested the Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT-P27) signaling pathway related to energy metabolism and found that the energy metabolism of ARPE-19 cells in the PAX6-shRNA group was slower than that of other groups (Figure 8). This result also suggests that the metabolic level of ARPE-19 cells is reduced after the onset of myopia, which may be caused by oxidative stress that brings some damage to ARPE-19 cells or there may be other reasons for abnormal cell metabolism, which may cause apoptosis if the process cannot be curbed, thus aggravating the progression of myopia to bring about a bad outcome, such as high myopia, retinal detachment or even blindness. Some studies have shown that cassia polysaccharides can participate in the PI3K/Akt pathway, affect the expression of B-cell lymphoma 2 (Bcl-2) to play an anti-apoptotic role inhibit the activation of Toll-like Receptor 4 (TLR4), reduce the expression of Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells (NF-κB), inhibit the TLR4/NF-κB signaling pathway and thus down-regulate the expression of inflammatory factors, such as Tumor Necrosis Factor alpha (TNF-α), Interleukin-1β (IL-1β), Interleukin-6 (IL-6) and Interleukin-18 (IL-18), which can play a preventive and protective role in hereditary ophthalmological diseases, such as retinitis pigmentosa [34,35].
Cassia seeds were first recorded in China's ancient medical book "Shennong Ben Cao Jing", its main bioactive substances are anthraquinones, fatty acids, naphthalene and pyrrolidone, polysaccharides and so on, with antioxidant, anti-aging, regulation of blood pressure and blood lipids, antibacterial and anti-inflammatory, inhibit obesity and other physiological effects. According to the Divine Husbandman's Classic of the Materia Medica, "Cassia seed is the main treatment for glaucoma, eye redness, swelling and pain, cataract and tearing". Cassia polysaccharides can scavenge O2-, -OH and HO2 produced during metabolism and reduce the production of peroxidation products MDA to enhance the antioxidant capacity of the body, thus preventing ocular diseases caused by oxidative damage and slowing down their pathogenesis [36]. This study only investigated the inhibitory effect of cassia polysaccharides on the development of myopia and further studies are needed to investigate the protective effects of other ophthalmic diseases and other components of cassia seed on ocular vision.
In summary, our study shows that the function of Wnt/β-catenin signaling pathway is over-activated in the state of myopia, which leads to the failure of myopia to improve and prolonged myopia and overexertion of the eyes will enter into a vicious cycle of vision loss. Cassia polysaccharides can alleviate the progression of myopia to some extent and also stop further scleral remodeling, but physical therapy or other more effective methods may be needed to completely improve the state of myopia.
The present study supports the view that the function of Wnt/β-catenin signaling pathway will be over-activated under long-term myopia and the over-activation of Wnt/β-catenin signaling pathway will lead to the lack of improvement of myopia, so the regulation of the normal expression of Wnt/β-catenin signaling pathway may become one of the potential methods to control the further development of myopia. And cassia seed polysaccharide can regulate the expression of Wnt/β-catenin signaling pathway to a certain extent, which provides a new perspective to control the development of myopia.
All data is provided within this manuscript or supplementary information files. Additional data relevant to this study can be obtained by contacting the corresponding author at any time.
Luo Jincheng and Qiu Hongbin conceived and designed the research. Luo Jincheng, Li Doudou, Sun Xuewei and Wang Xue engaged in experimental operations. Luo Jincheng and Li Doudou analyzed the data of the entire work. Luo Jincheng wrote the manuscript. Li Peixuan revised the draft. All authors have checked the final manuscript, contributed to the article and approved the submitted version.
This work was financially supported by Heilongjiang Provincial Colleges and Universities Basic Scientific Research Projects, China (2020-KYYWF-0300)
We sincerely thank each of the authors for their contribution to this study also thank the northern medicine and functional food characteristic discipline program of Heilongjiang province for their help.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Jincheng L, Doudou L, Peixuan L, Jialin L, Xuewei S, Xue W, et al. (2024). Cassia Polysaccharide Regulates the Effect of PAX6 Low Expression on Myopia through the Wnt/β-Catenin Pathway. J Clin Exp Ophthalmol. 15:995.
Received: 29-Oct-2024, Manuscript No. JCEO-24-35308; Editor assigned: 31-Oct-2024, Pre QC No. JCEO-24-35308 (PQ); Reviewed: 14-Nov-2024, QC No. JCEO-24-35308; Revised: 22-Nov-2024, Manuscript No. JCEO-24-35308 (R); Published: 29-Nov-2024 , DOI: 10.35248/2155-9570.24.15.995
Copyright: © 2024 Jincheng L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.