Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2016) Volume 7, Issue 1
Microalgae are very promising organisms for the production of high-value compounds such as carotenoids. Nevertheless, their commercial use is so far hampered by the lack of efficient processes and currently feasible for very few strains. One of the most relevant factors is the high effort for downstream processing, e.g., cell disruption and extraction. Thus, the presented studies were dedicated to investigate and optimize these two steps for carotenoid production with microalgae. Six different cell disruption techniques (high pressure homogenizer, ball mill, Ultra Turrax, repeated freeze and thaw, freeze-drying, ultra-sonication) were compared in lab scale for three species: Haemotococcus pluvialis, Chromochloris zofingiensis and Chlorella sorokiniana. The carotenoid recovery was determined via HPLC-UV/Vis after pressurized solvent extraction. Furthermore, factors influencing the applied extraction methods such as solvent, temperature, duration and number of cycles were optimized in order to reach highest recovery rates. While rough mechanical methods such as ball mill and high pressure homogenizer showed the highest effectivity for cell disruption of all three investigated strains, the influence of non-mechanical methods - i.e., repeated freeze and thaw cycles - on the efficiency of the extraction of astaxanthin, lutein and β carotene increased reversely proportional to cell size and cell wall rigidity. For H. pluvialis repeated freezing and thawing resulted in a factor 240 times lower extraction yield compared to high pressure homogenizer, while both methods were comparable for C. sorokiniana. From six tested solvents, dichloromethane resulted in the highest carotene recovery yield, three times higher in comparison to n-hexane. Variation of the extraction temperature from room temperature to 120°C showed an optimum at 60°C. Nearly complete extraction was reached after one cycle of 10 minutes. The data presented here demonstrate the necessity of lab scale optimization of cell disruption and extraction process for future upscaling
Keywords: H. pluvialis; C. sorokiniana; C. zofingiensis; Carotenoids; Cell disruption; Pressurized liquid extraction; HPLC
C: Cell Disruption Yield; CDW: Cellular Dry Weight; DCM: Dichloromethane; DMSO: Dimethyl Sulfoxide; HPLC: High Performance Liquid Chromatography; MeOH: Methanol; MTBE: Methyl tert-Buthyl Ether; PFD: Photon Flux Density; PLE: Pressurized Liquid Extraction; PSM: Photobioreactor Screening Module; SAG: Culture Collection of Algae at Goettingen University in Germany
Microalgae, including cyanobacteria, play an increasing role in science and industry, due to their wide range of commercial and potential novel products [1-3]. They can be cultivated using sunlight (energy source) and waste streams, such as recycled media from other processes (e.g., aquaculture, biogas digestate - nutrient source) and industrial flue gas (anorganic carbon source), that positively contribute to cost reduction and ecological balance [4-9]. One of the most important, and on the market best established, high value products from microalgae are carotenoids. Carotenoids are a group of structurally highly diverse terpenoid pigments (more than 750 have been isolated), which can be divided into carotenes and oxygenated derivatives of carotenes, so called xanthophylls [10-12]. They act as light-harvesting pigments, absorb light in a range of λ=400-550 nm and transfer the light to chlorophyll [12,13]. Furthermore, they stabilize the structure and the functionality of the photosynthetic apparatus [11,12], e.g., by quenching chlorophyll triplet states, dissipating excess energy, or scavenging reactive oxygen species [11,14]. In general, applications of carotenoids can be divided into three main groups: i) as natural dyes in food and feed industry, ii) as feed additives in aquaculture and poultry farming, iii) as well as in the pharmaceutical sector and in cosmetics, due to their antioxidative properties [10,11,15]. The most commonly used carotenoids are β-carotene, astaxanthin and lutein that can be produced by various microalgae such as Dunaliella salina, Haematococcus pluvialis, Chromochloris zofingiensis, Chlorella sorokiniana, etc. [1,11,15]. The overall market for carotenoids was estimated to US$ 1.2 billion in 2010 and was predicted to increase up to US$ 1.4 billion in 2018 [15]. Market prices vary from 300 - 3,000 US$â??kg-1 and 2,500-10,000 US$â??kg-1 in case of β-carotene and astaxanthin, respectively [16,17].
Although there are many described algae species, until now only few have found application in biotechnological processes (e.g., H. pluvialis, D. salina, Arthrospira platensis, Chlorella species) [18]. This is due to still relatively high production costs making them incapable of competing with the chemical industry while only delivering one class of value products [19]. To approach this issue, integrated processes allowing almost complete utilization of the produced biomass, waste streams and nutrients are needed. Derived from the oil-based refinery, in which the raw material is more or less utilized completely, such integrated biotechnical applications are called biorefinery processes. Particularly, an optimal design of the downstream processing is essential for feasibility of such systems as it may contribute up to 90% of the total costs [20].
In order to establish an efficient isolation procedure for potential products for the future industrial application, at first an optimization of the extraction in labscale has to be performed for various strains of microalgae. This is not only necessary during the screening phase to assure comparability of the results, but also in later stages of the process design as the collected data and experience can be used for example for upscaling. In case of intracellular products not only extraction parameters such as: selected solvent, temperature, duration, cycle number are crucial but also an efficient cell disruption. At the same time, when considering further upscaling of the process, other aspects such as costs and feasibility for industrial application have to be considered. Thus, the main aim of the work presented here was the optimization of the cell disruption and extraction procedure for carotenoids for future application as an important isolation step of one of the relevant product classes in a microalgae based biorefinery.
Strains and media
In this work, three microalgae strains, provided by the Culture Collection of Algae at Goettingen University in Germany (SAG), were used: Haematocouccus pluvialis SAG 34-1b, Chromochloris zofingiensis SAG 211-14 and Chlorella sorokiniana SAG 211-8k. Basal medium (with 1 gâ??L-1 peptone and without soil extract) suggested by SAG was used for photoautotrophical cultivation of H. pluvialis. In case of C. zofingiensis and C. sorokiniana, a modified medium of Arnon et al. [21] described by Cordero et al. [10], was used for photoautotrophical cultivation.
Cultivation
Photobioreactor screening modules (PSM) were used for cultivations under monoseptic conditions, as previously described [22-24]. The aeration was kept constant at 0.45 Lâ??min-1, enriched with 3% CO2, and the temperature was 25°C. The light intensity (photon flux density PFD) was measured with a submersible spherical light sensor (US-SQS/L, ULM-500, Walz, Germany) in the center of the PSM filled with water. H. pluvialis and C. sorokiniana were cultivated with a light intensity of 700 μmol·m-2·s-1. In case of C. zofingiensis the PFD was increased after six days from 470 up to 700 μmol·m-2·s-1 to boost the carotenoid production. All three microalgae were kept in batch mode for two weeks.
Determination of algae carotenoid yield
After two weeks of cultivation, the harvested and freeze-dried biomass was disrupted/homogenized using various methods described below and extracted via pressurized liquid extraction (PLE) at the given conditions. Extracts were evaporated to dryness under the nitrogen and redissolved in 2.5 mL of a mixture of acetone and MeOH (9:1, v:v) before saponification. The latter was started by addition of 0.5 mL of 0.05 M NaOH in MeOH and incubated for 3 h (room temperature, darkness) to eliminate carotenoid esters [25-27]. Next, 3 mL of petroleum ether were added, the mixture was washed with 3 mL 10% NaCl, centrifuged (3,500 rpm, 1 min, 5810R, Eppendorf; Hamburg, Germany) and the aqueous phase was discarded. The washing step was repeated twice. The resulting organic phase was evaporated to dryness under N2 and the resulting substance was redissolved in 1 mL of MTBE. Extracts were analyzed via HPLC-UV/Vis (Prominence HPLC System, Shimadzu, Japan) according to conditions reported previously by Sander with the following modifications [28]. The chromatographic separation was performed on a reversed phase column (YMC carotenoid C30, 150 × 4.6 mm, 3 μm) with a mobile phase consisting of two solvent mixtures of methanol, MTBE and water (A: 81:15:4, B: 8:89:3, v:v:v) under following gradient conditions. The concentration of 2% B was kept constant for 11 minutes, then increased to 40% B in 7 minutes and held for 6.5 minutes, followed by an increase to 100% B (in 2.5 min, held for 3 min) and successive reequilibration to 2% B (in 3 min, held for 7 min). The flow rate was 1 mL·min-1, injection volume was 5 μL and column temperature was kept at 22°C. The detection of the analytes was done at 445, 452 and 475 nm for lutein, beta-carotene and astaxanthin, respectively. All these steps were performed in triplicates.
For calibration, a stock solution of astaxanthin (Adipogen International Inc., San Diego, USA) was prepared by dissolving 10 mg in 33.3 mL of DMSO, resulting in a concentration of 300 μg·mLâ??1. Stock solutions of lutein (CaroteNature GmbH, Ostermundigen, Switzerland) and β-carotene (Sigma Aldrich, St. Louis, USA) had the same concentration by solving 1 and 10 mg in 3.3 and 33.3 mL of MTBE, respectively. All analytes were calibrated using mixed standards prepared by appropriate dilution of stock solutions in a range of 0.05 to 100 μg·mLâ??1.
Cell disruption methods
In the scope of this work various cell disruption methods were evaluated based on the optical determination of the disruption yield and total carotenoid yield after pressurized liquid extraction (Dionex ASE 350, 1 cycle, 30 min, 100 bar, 60°C, solvent:acetone:MeOH, 9:1 v:v for H. pluvialis and DCM for C. zofingiensis and C. sorokiniana). The disruption yield C was calculated as ratio of intact and disrupted cells by counting in a Neubauer‑improved hemocytometer (equation 1).
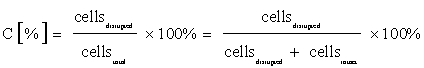
The following disruption methods, performed according to the parameters summed up in Table 1, were compared: labscale high pressure homogenizer (French Pressure Cell Press, SLM instruments, Inc., Urbana, USA), labscale ball mill (TissueLyser LT, Qiagen, Venlo, Netherlands), Ultra Turrax (T 18 Basic, IKA-Werke, Staufen, Germany), repeated freeze and thaw cycles, freeze-drying (Alpha 1-4, Christ, Osterode, Germany) and ultra-sonication (UW 2200, Bandelin electronic, Berlin, Germany). All samples were freeze-dried again before extraction in order to remove water residues and assure comparability. The methods were tested on freeze-dried biomass of three algae with different morphological characteristics: (I) H. pluvialis (30 mg, encysted state, 30-50 μm, rigid cell walls, [29,30], (II) C. zofingiensis (30 mg, 2â??15 μm, [31]) and (III) C. sorokiniana (30 mg, 2-4. μm, [32]). The optical evaluation of the disruption yield was exemplarily applied on H. pluvialis.
| Cell disruption | Parameters |
|---|---|
| High pressure homogenizer | Biomass resuspended in 10 mL of H20u, p=1000 bar, cycles n=3 |
| Ball mill | Biomass, frozen at -80°C for 1 h, 1 cycle, 50 Hz, 2 min |
| Freeze and thaw | Biomass resuspended in 10 mL of H20u, freezing -20°C, thawing at RT for 30 min, cycles n=3 |
| Freeze-drying | Biomass resuspended in 10 mL of H20ufrozen at -80°C overnight, P=0.4 mbar, overnight |
| Ultra-sonication | Biomass resuspended in 10 mL of H20u, maximum intensity, 10 min, one cycle |
| Ultra Turrax | Biomass resuspended in 10 mL of H20u, 24,000 rpm, 10 min, on ice |
Table 1: Parameters for various cell disruption methods tested. Each method was performed with dried biomass of H. pluvialis (30 mg), C. zofingiensis, C. sorokiniana (40 mg).
Optimization of the pressurized liquid extraction method
Selection of the solvent for PLE of carotenoids from algae biomass (30 mg dried biomass, 1 cycle, 30 min, 100 bar, 60°C) was performed based on the experiments with biomass of H. pluvialis after Ultra Turrax-disruption (conditions see Table 1) using 6 different solvents: acetone, ethanol, ethyl acetate, nâ??hexane, dichloromethane and a mixture of acetone:MeOH (9:1, v:v).
Next, the influence of temperature on the efficiency of the carotenoid extraction at first between 20 and 60°C (1 cycle, 30 min, 100 bar, solvent: acetone:MeOH, 9:1, v:v) and later between 60 and 120°C (1 cycle, 30 min, 100 bar, solvent: DCM) was tested with the biomass of C. zofingiensis (30 mg, freeze-dried, no disruption) and H. pluvialis (40 mg, after Ultra Turrax-disruption).
After appointing the optimum extraction temperature, various extraction durations - 5, 10, 20 and 30 min - were investigated, on the same biomass of H. pluvialis that was used before, in order to test the possibility of shortening the extraction time for increased sample throughput. Additionally, the effect of using multiple extraction cycles (4 × 5 min) was evaluated.
Cell disruption
A downstream process for recovery of intracellular products in general consists of the following unit operations: harvesting, dehydrating, product isolation (cell disruption and extraction) and product purification [18]. To gain high product yields, both efficient cell disruption and extraction is necessary. For instance in the work of Ceron et al. the recovery of lutein from freeze-dried biomass of S. almeriensis with various cell disruption methods was up to 60% higher compared to a method without cell disruption [33]. Cell disruption can be performed by a multitude of established methods, generally divided into non-mechanical methods, such as alkaline lysis, organic solvents, freezing, osmotic shock, etc. or mechanical methods, e.g., homogenizers, bead mills, ultra-sonication [18,34].
In the work presented here, four mechanical (ball mill, Ultra Turrax, high pressure homogenizer and ultra-sonication) as well as two nonmechanical methods (repeated freeze and thaw cycles, lyophilization) were used for the three microalgae H. pluvialis, C. zofingiensis, C. sorokiniana. To evaluate the efficiency of the mentioned methods an optical determination of the disruption yield C, based on counting disrupted and intact cells after cell disruption, was used (see equation 1). The disruption yield C of freeze-dried H. pluvialis biomass is shown in (Figure 1). The highest disruption yield - around 80% - of the thick encysted cell walls was reached by using mechanical methods such as ball mill, Ultra Turrax and high pressure homogenizer, while other mechanical (ultra-sonication) and non-mechanical methods, such as repeated freeze and thaw cycles resulted in disruption yields of ≤ 10%. In the next step the disrupted biomass was used for determination of the total carotenoid extraction yield via HPLC-UV/vis analysis. The obtained total carotenoid yield values are normalized to the highest reached value for each alga (in case of H. pluvialis - high pressure homogenizer) (Figure 2). In general, the normalized total carotenoid values are in good agreement with the calculated disruption yield. The highest carotenoid extraction yield was measured after disruption with the high pressure homogenizer (4.21 μg total carotenoid·mg-1 dry weight (d.w.), taken as 1 for normalization purpose), ball mill (3.56 μg total carotenoid·mg-1 d.w.) and Ultra Turrax (3.42 μg total carotenoid·mg-1 d.w.). Repeated freeze-and-thaw-cycles resulted in 240-fold lower total carotenoid concentration (0.02 μg total carotenoid·mg-1 d.w.) in comparison to high pressure homogenizer. Similar observations for H. pluvialis are described in the work of Mendes-Pinto et al. In this work mechanical disruption via a cell homogenizer resulted in nearly factor four higher total carotenoid amounts compared to nonmechanical methods like alkaline lysis, spray drying or treatment with base or enzymes. Also autoclaving of the biomass showed nearly the same effectivity as mechanical disruption [35]. However, even though the method used here for the determination of the disruption yield gives a very fast and a relatively good estimation of the disruption effectivity, it can only be performed with the statistically selected parts of the samples and only for some algal species. In case of H. pluvialis, the method can be easily applied and gives reliable data due to big cell diameters during encysted stadium [36]. For the other two investigated microalgae C. zofingiensis and C. sorokiniana with small cell diameters, it is inapplicable. Furthermore, the optical impression of the cell integrity should not lead to the conclusion that carotenoids cannot be extracted, as minor damage might not be visible but might still allow extraction of carotenoids. All in all, this method used here for H. pluvialis is a quick and easy one and results in a rough estimation, however due to the above listed limitations, further evaluation based on the total carotenoid yield measured by HPLC is necessary.
Figure 2: Normalized cumulated carotenoid yield of H. pluvialis, C. zofingiensis and C. sorokiniana measured by HPLC-UV/Vis resulting from different cell disruption methods (black bar: high pressure homogenizer; light gray bar: ball mill; white bar: Ultra Turrax; dashed bar: repeated freeze and thaw; double dashed bar: freeze-drying; dotted bar: ultra-sonication; n.d.: not determined). Freeze-dried algae biomass (30, 40 and 40 mg for H. pluvialis, C. zofingiensis and C. sorokiniana) was disrupted as described, extracted via PLE (60°C, 30 min, 100 bar) with acetone:MeOH (9:1, v:v) (H. pluvialis) or DCM (C. zofingiensis, C. sorokiniana), saponified and analysed by HPLC-UV/Vis on a C30 column. Data was normalized on the highest value reached for each alga.
In the next step, the best three disruption methods selected in the experiments with H. pluvialis were tested on the biomass of C. zofingiensis (2-15 μm), showing the best results again for the high pressure homogenizer (2.87 μg total carotenoid·mg-1 d.w.) and ball mill (2.81 μg total carotenoid·mgâ??1 d.w.). In contrast to the other two species, for C. sorokiniana - the smallest tested cells (2-4.5 μm) - the effectivity of repeated freeze and thaw cycles of the biomass showed comparable results (1.95 μg total carotenoid·mg-1 d.w.) to the results reached with the use of high pressure homogenizer (1.97 μg total carotenoid·mg-1 d.w.) and ball mill (2.15 μg total carotenoid·mgâ??1 d.w.). Furthermore, also other methods, such as lyophilization and ultra-sonication, showed better results for this species (0.57 and 0.31 normalized carotenoid yield) in comparison to H. pluvialis (0.005 and 0.08 normalized carotenoid yield) (Figure 2). This is in good correspondence to the data of Prakabaran et al. that showed comparably good results achieved by osmotic shock and bead mill for lipid extraction for Chlorella sp. [37]. As a consequence, it can be assumed that the effectivity of nonmechanical disruption methods increases inversely proportionally to the cell size and the rigidity of cell walls of the examined species.
In order to establish an efficient isolation procedure for industrial application, the suitability of the various investigated methods for scale-up has also to be taken into account. Because of high power consumption per unit of biomass volume ultra-sonication and lyophilization are considered to be suitable only for small-scale applications [18,38]. Capital cost for repeated freeze and thaw cycles are relative low compared to the other used methods. However, the fact that this is a very time-consuming as well as the difficulty to maintain the product quality under such conditions has to be addressed. In contrast to this, mechanical methods i.e., high pressure homogenization and bead mills are commercially available and applied [18]. Furthermore, these methods showed the best disruption yields resulting in the highest carotenoid recoveries for all three tested algae. Finally, the right choice of the best cell disruption method strongly depends on the used microalgae, the product of interest as well as the scale of production (quantity and quality) [18,34].
Pressurized liquid extraction of carotenoids
One of the most important factors affecting the extraction yield of carotenoids via PLE is the selection of the suitable extraction solvent (Figure 3). The application of n-hexane for H. pluvialis resulted in the isolation of the lowest measured carotenoid amount (1.59 μg·mg-1 d.w.), followed by ethyl acetate (1.98 μg total carotenoid·mg-1 d.w.), ethanol (2.15 μg total carotenoid·mg-1 d.w.), acetone (2.43 μg total carotenoid·mg-1 d.w.) and acetone:methanol (9:1, v:v) (3.43 μg total carotenoid·mg-1 d.w.). The use of DCM showed the best results with 4.72 μg total carotenoid·mg-1 d.w. - a 3-folds higher yield than reached with hexane. However, this general finding should not be generalized for individual carotenoids. In fact, βâ??carotene gave comparable results for most applied solvents whereas the yield for lutein extraction could be improved by factor 2.9 and for astaxanthin by factor 3.2 by using DCM compared to hexane. This can be explained by the increased solubility of more polar compounds such as astaxanthin or lutein in dichloromethane (i.e., astaxanthin 30 g·L-1 in DCM, 0.2 g·L-1 in acetone [27]). Increased solubility of the very unpolar β-carotene in hexane (30 mg Lâ??1, described by Craft and Soares) compared to the other solvents did not show significant influence [39]. Due to the low yield in the examined species (0.2 mg Lâ??1 in the extract) all tested solvents were able to extract it completely. Jamie et al. also reported better extraction yields for PLE extraction from H. pluvialis using ethanol in comparison to n-hexane, which is in consent with the results presented above [40]. Mixing dichloromethane with other solvents showed no additional advantages (data not shown here), while Denery showed the best results for a mixture of DCM and methanol (1:3, v:v) for extraction of astaxanthin from H. pluvialis compared to acetone, ethanol and mixtures of acetone and methanol (7:3, v:v) [41]. In contrast to this, various mixtures of acetone and methanol are commonly used in literature as extraction solvents [10,25,42]. Furthermore, other extraction media such as oil [43,44] or CO2 for supercritical fluid extraction [45,46] have also been described in literature but not tested in this work. Further tests are necessary to evaluate these methods.
Figure 3: Yield of individual and total carotenoids (black bar: ß-carotene; light gray bar: astaxanthin; dark gray bar: lutein; dashed bar: total carotenoids) ofH. pluvialis measured by HPLC UV/Vis resulting from pressurized liquid extraction with different solvents: 30 mg of freeze-dried H. pluvialis biomass were disrupted using Ultra Turrax, extracted via PLE (60°C, 30 min, 100 bar) by six selected solvents, saponified and analysed by HPLC -UV/Vis on a C30 column.
The first experiments with temperature variations for PLE showed that the yield of the carotenoid extraction can be improved by increasing the temperature from room temperature to 60°C. Therefore, further tests with even higher temperatures were conducted here in order to check if additional improvement is possible, as the solubility itself is a thermodynamic equilibrium, influenced by the temperature. It turned out, that the carotenoid recovery yield for the selected solvent decreased again with temperatures above 80°C, probably due to the thermal destruction of the analytes (Figure 4). At 120°C, the total yield of carotenoids was 35% lower than after extraction at 60°C (3.08 compared to 4.72 μg total carotenoid·mg-1 d.w.). The optimum could be found around 60°C. That corresponds well with findings of Mustafa et al. who tested PLE with ethanol for α- and β-carotene from carrots showing 42% reduction of β-carotene recovery by using 180°C in comparison to 60°C [47]. Denery et al. did not find a reduction of astaxanthin recovery by PLE from H. pluvialis using acetone between 20°C and 100°C but a reduction of lutein and total pigment amounts at higher temperatures [41]. In contrast to this, Jaime et al. found increasing extraction yields for temperatures up to 200°C for carotenoid extraction with ethanol from H. pluvialis [40]. From the somewhat contradicting results reported by the above mentioned groups, it can be assumed that optimum temperature depends on the solvent that is used for the extraction and has to be carefully selected for specific applications. In the case of the work presented here, future tests with smaller temperature intervals might reveal a more exact optimum.
Figure 4: Yield of individual and total carotenoids (black bar: ß-carotene; light gray bar: astaxanthin; dark gray bar: lutein; dashed bar: total carotenoids) ofH. pluvialis measured by HPLC UV/Vis resulting from pressurized liquid extraction with different temperatures: 30 mg of freeze-dried H. pluvialis biomass were disrupted using Ultra Turrax, extracted via PLE (30 min, 100 bar, DCM) by 4 different temperatures, saponified and analysed by HPLC -UV/Vis on a C30 column.
The optimum duration of the extraction process depends on the time needed to build up the equilibrium between the analyte concentration in the sample matrix and the solvent. Five minutes showed to be insufficient for carotenoid extraction in the tested setup, resulting in only 79% of the maximal total carotenoid yield (3.74. instead of 4.72 μg total carotenoid·mg-1 d.w.) and 58% of the lutein recovery (0.63 instead of 1.08 μg lutein·mg-1 d.w.), whereas a nearly complete carotenoid level could be reached applying 10 minutes and more (Figure 5). Implementing additional extraction cycles did not improve the results, as 99% of the analytes were extracted within the first cycle (Figure 6). The carotenoid yield in the extracts of the third and fourth cycle were below the detection limit (0.0125 μg·mgâ??1 for astaxanthin and lutein, 0.025 μg·mgâ??1 for ß-carotene). This suggests that the equilibrium between solvent and biomass is established after 10 minutes and according to the solubility data was also far away from saturation [27,39]. Performing multiple steps is often preferred in literature, e.g., Mustafa et al. showed that the optimum PLE of β-carotene with ethanol at 60°C to be five 2 min cycles after 5 min preheating [47]. On the other hand, this highly depends on the solvent and the used matrix.
Figure 5: Yield of individual and total carotenoids (black bar: ß-carotene; light gray bar: astaxanthin; dark gray bar: lutein; dashed bar: total carotenoids) ofH. pluvialis measured by HPLC UV/Vis resulting from pressurized liquid extraction with different extraction durations: 30 mg of freeze-dried H. pluvialis biomass were disrupted using Ultra Turrax, extracted via PLE (60°C, 100 bar, DCM) for 4 different durations, saponified and analysed by HPLC -UV/Vis on a C30 column.
Figure 6: Yield of individual and total carotenoids (black bar: ß-carotene; light gray bar: astaxanthin; dark gray bar: lutein; dashed bar: total carotenoids) ofH. pluvialis measured by HPLC UV/Vis resulting from pressurized liquid extraction with multiple extraction cycles: 30 mg of freeze-dried H. pluvialis biomass were disrupted using Ultra Turrax, extracted via PLE (60°C, 10 min, 100 bar, DCM) for 4 different durations, saponified and analysed by HPLC -UV/Vis on a C30 column.
With the parameters selected (high pressure homogenizer, PLE at 60°C for 10 min, in 1 cycle, solvent: DCM), a total carotenoid yield of 4.78 μg total carotenoid·mg-1 d.w. and a total astaxanthin yield of 3.69 μg astaxanthin·mg-1 d.w. could be determined for H. pluvialis, which is 0.4% of the total cell weight. The values that can be found in literature range from 0.5 to 4% astaxanthin depending on the strain, culture conditions and cultivation mode [31,34,41,42,48]. Values of up to 98 μg·mg-1 d.w. have been reported for heterotrophic cultivations [49]. The lower carotenoid yield presented in this study can be easily explained by the fact that no optimization regarding the carotenoid production was performed at this stage of the work. As numerous possible modifications of the process to induce astaxanthin production is described in literature (nitrogen starvation, light stress, salinity, iron addition, strain selection, genetic modifications, heterotrophic growth on acetate), further optimizations of the cultivation is possible and planed for future work [50-56]. High pressure homogenizer and ball mill showed the best disruption for C. zofingiensis, the parameters for the PLE were based on the findings regarding the extraction of carotenoids from H. pluvialis. Applying an optimized isolation process for carotenoids to the biomass of C. zofingensis 1.48 μg lutein·mg-1 d.w. and 1.29 μg astaxanthin·mg-1 d.w. could be measured. Values reported for this species by Del Campo and Liu range between 4 and 1.3 to 6.5 μg·mg-1 d.w. for lutein und astaxanthin, respectively [25,31]. The lower yield for lutein measured in this work can be explained by the harvesting of the biomass at the end of the cultivation while the highest yield was reported for an early stage of cultivation by Del Campo et al. [25]. Thus further optimization of the whole production process including cultivation is necessary. Possible approaches were published in literature [50,57,58]. For C. sorokiniana the highest yield of lutein (2.08 μg lutein·mg-1 d.w.) was measured after the ball mill disruption. In comparison, Cordero et al. reported 4.2 μg lutein·mg-1 d.w. in wildtype algae and up to 7 μg·mg-1 d.w. in genetically modified strains, also suggesting that further optimization of the cultivation process is necessary [10].
Due to the fact that downstream processing is one of the decisive elements, it has to be very carefully optimized in order to reach the maximum recovery yield of the products at lowest possible costs. For this reason, various cell disruption methods and parameters for pressurized liquid extraction of the carotenoids from various algae species were examined in labscale in this work. The influence of the cell size and cell wall properties on the success of cell disruption methods was shown: while mechanical methods, i.e., high pressure homogenizer or ball mill were most efficient for all three tested microalgae, the effectivity of soft disruption increased with decreasing cell size and rigidity. Selecting DCM as the extraction solvent at 60°C improved the extraction yield of total carotenoids. The extraction time could be set to 10 minutes, with no additional extraction cycles, improving the sample throughput. The best extraction yield for carotenoids from above described species could be achieved by PLE using DCM at 60°C in a 10 min cycle (n=1) with lyophilized and high pressure disrupted biomass. The carotenoid yield was lower than the highest values reported in literature since when this work was carried out the cultivation conditions were not optimized for the highest product yield. Obviously, culture optimization will be as crucial as optimizing the downstream process. Nevertheless, the proposed sample preparation procedure allows reliable carotenoid determination in algae biomass that is necessary for further optimization of the whole process for economic use of microalgae for this application.
The authors have declared no conflicts of interest.
The authors are grateful for financial support of the Federal Ministry of Education and Research (BMBF) and the Bavarian State Ministry of the Environment and Consumer Protection, due to which this work could be performed.