Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2020)
Introduction: Hepatitis B Virus (HBV) infection is a global public health concern. The immune response in HBV represents a key factor in patient outcome. However, the relationship between viral replication and host immune reactivity remains a matter of investigation.
Aim: The aim of our study was to investigate whether the cellular immune response of recently diagnosed and treatment naïve chronic hepatitis B(CHB) patients may be influenced by the replicative status of HBV. Towards this aim, the correlation between HBV viral load, HBsAg quantification and peripheral T-cell subpopulations CD8+CD38+.
Methods: Proportions and absolute counts of CD8 CD38 T cells were determined using three-color flow cytometry in chronic hepatitis B patients (n=50) and healthy controls (n=35). Chronic hepatitis B patients were regularly followed for 48 weeks, during which period the T cell subsets, serum viral load and HBsAg quantitation were measured every 24 months.
Results: There was a high level of CD8+CD38+% during the pretreatment stage (Mean 32.4514, standard deviation (SD) 16.8007) compared to the control group (Mean 19.4628, SD 9.75555), p=0.000. Significant decreases in CD8 count were detected 12 months after treatment initiation of HBV therapy (Mean 1359.44, SD 724.362) compared to the control group (Mean 1944.13, SD 948.931), p=0.001. There is a significant correlation between CD8+CD38+ count and serum HBV DNA. A Positive correlation was found between CD8+CD38+ count and HBsAg quantitation.
Conclusion: There was a positive correlation between CD8+CD38+ T cells and HBsAg quantitation. The combined use of CD8+CD38+ T cells, HBsAg quantitation and HBV DNA assessment in patients with CHB may guide the clinicians as they guage the likelihood of treatment response.
Hepatitis B; CD8; CD38; HBsAg quantitation
Hepatitis B virus (HBV) infection is a global public health concern. It has been estimated that 350 million patients are infected with HBV worldwide [1]. Chronic HBV infection may lead to severe sequelae such as liver fibrosis, cirrhosis and hepatocellular carcinoma [2]. Nearly 1 million people die every year from acute or chronic sequelae of primary infection with HBV. In Egypt, 75%-85% of patients with chronic liver disease have HBV or hepatitis C virus (HCV) infection as a contributing cause [3].
HBV replication itself is not directly cytotoxic to cells, as evidenced by the large numbers of asymptomatic HBV carriers who have minimal liver injury, despite ongoing intrahepatic replication of the virus. The immune responses to HBV antigens are responsible both for viral clearance during acute infection and for disease pathogenesis. In infected humans, viral clearance follows the development of a vigorous immune response associated with acute, self-limited inflammatory liver disease (i.e, acute viral hepatitis) [4].
Clinical outcomes of HBV infection largely depend on the quality and strength of the host’s immune response. Studies have revealed that T cellular immune responses are essential for disease pathogenesis [5], and have identified CD8+ T lymphocytes as the main cellular subset responsible for viral control [6]. Compared with acute self-limiting infection, lack of vigorous and multi specific T cell response in chronic HBV infection has been observed, which leads to the failure of viral clearance and the progression of the disease [7]. The composition of peripheral T cell subpopulations, on the other hand, serves as a valuable index for evaluating T immune status in chronic HBV infection. Impaired balance of peripheral T subpopulations has been reported at various stages of chronic HBV infections, associated with HBV replication levels, and can be partially restored with antiviral therapy [8]. However, results from previous studies are controversial regarding the specific changes arising during chronic HBV infection, and few have gone so far as to investigate the dynamics of particular T cell subpopulations amid antiviral treatment. The abnormal activation of CD8+ T cells in chronic HBV infection can be partially reversed by antiviral therapy. HBV-associated immune activation may be a crucial part of pathogenesis and may provide a promising target for treatment [9].
CD38 is a surface glycoprotein existing on many immune cells. It is ubiquitously expressed on lymphocytes in a unimodal distribution [10,11]. In certain infections, CD8+CD38+ T cells undergo a rapid up-and-down pattern after the infection once the immune control of the acute phase is achieved [12]. However, persistency of immune activation and CD38+ expression throughout the acute and chronic phase is possible, which may reflect the failure of the host’s immune system to fully suppress viral replication. With some infections, a correction of CD38 expression level could also be observed soon after effective treatment, (e.g., HIV, EBV infections) [13,14]. Whether it is the same case with chronic HBV infection deserves more exploration. Quantitating the mean intensity of CD38 expression on CD8 T cells rather than just the proportion of positive cells is preferable and better reflects the continuous distribution of CD38 on CD8 T cells [15].
The Hepatitis B surface antigen (HBsAg) was identified more than 50 years ago [16]. Detection of HBsAg in serum is still the hallmark of HBV infection and the cornerstone for the diagnosis of chronic hepatitis B (CHB). Over the years, HBsAg quantification has proved to be a reliable marker for predicting clinical outcome [17]. HBsAg quantification was considered to be a promising, simple, and inexpensive method to monitor viral replication in CHB patients. Studies have reported that the management of CHB can be optimized in daily practice using HBsAg quantification [18]. Previous studies have suggested that HBsAg may reflect the content of intrahepatic HBV covalently closed circular DNA (cccDNA), which has been proposed as a surrogate marker of HBV-infected hepatocytes. HBsAg indirectly represents cccDNA activity and viral replication that could also be representative of immune response [19].
The present study is designed to elucidate the correlation between HBV viral load, HBsAg quantification, and peripheral T- cell subpopulations CD8 and CD38.
Subjects
Fifty chronic HBV infection patients and 40 healthy controls were recruited from Egyptian Liver Research Institute and Hospital (ELRIAH), Mansoura, Egypt, from June 2013 and February 2014.
All patients fulfilled the following criteria: positive HBsAg, no other concomitant causes of liver disease (Hepatitis C and D, HIV infection, autoimmune hepatitis and metabolic liver disease). None of the patients were drug users or exposed to hepatotoxin. All patients gave their informed consent to participate in the study. The study protocol was approved by the Institutional Review Board of ELRIAH (IORG #66187).
All patients were assigned to hepatitis B monotherapy based on their clinical manifestations. All treated patients were followed for a minimum of 24 and 48 weeks after treatment initiation. Virological, and biochemical assessments, as well as T cell subset measurements were carried out before treatment and at each point of follow-up.
Biochemical tests of liver function and evaluation of HBV markers
Serum Alanine aminotransferase (ALT), Aspartate transaminase (AST), Albumin and total bilirubin levels were measured with routine automated systems COBAS INTEGRA 400 analyzer (Roche Diagnostics, Switzerland).
HBV markers (HBsAb, HBeAg, HBeAb, HBcAb, and HBsAg) were detected by automated system ARCHITECT (ABBOTT- Germany).
HBsAg quantitation ARCHITECT QT assay (Abbott Diagnostic, Wiesbaden, Germany) according to the manufacturer’s instructions, the assay was carried out in two steps: HBsAg present in the sample was bound to antibody to hepatitis B surface antigen (anti-HBs)-coated microparticles, and an acridinium- labeled anti-HBs conjugate was added together with pretrigger and trigger solutions. The products of the resulting chemiluminescent reaction were measured in relative light units. The qHBsAg calibration curve ranged from 0.05 to 250 IU/mL, and the samples were diluted with a diluent (1: 20 or 1: 250) as needed to expand the detection range.
Detection of HBV DNA
Serum HBV DNA load in individuals was detected by real time polymerase chain reaction (RT-PCR) using fully automated CobasAmpliprep/Taqman 48 (ROCHE diagnostics GmbH, Germany) with a lower detection limit of 15 IU/ml with a reagent kit package insert (CobasAmpliprep/Taqman HBV quantitative test, v2.0, ROCHE diagnostics GmbH, Germany) following the manufacturer's instructions. The primer was provided with the kit. The reaction volume was 100 μl. PCR was performed at 50°C for 2 min and at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and at 57°C for 1 min.
Detection of peripheral blood T lymphocyte subset (CD8+CD38+)
CD8+CD38+ were measured routinely at each follow-up assessment. Count and percentage of CD8+CD38+ were determined on 100 μL ethylenediamine tetra-acetic acid (EDTA) blood sample, using a three-color MACS quant flow cytometer (Miltenyi Biotec, Germany). In sample tubes CD8 cells were autogated and analyzed for CD38 expression using anti-human monoclonal antibodies, CD8- APC/CD38-FITC. Absolute counts of lymphocyte subpopulations were calculated according to complete blood count on the same day.
Statistical analysis
The statistical analysis of data was performed using Excel (Microsoft Office 2013) and SPSS (statistical package for social science) program (SPSS, Inc, Chicago, IL) version 21.
Quantitative data were presented by mean and standard deviation (SD). Comparisons between two groups were completed using a t-test. A paired sample t-test was used to compare quantitative data from different visits. Correlations were determined using Pearson’s correlation. p-values less than or equal to 0.05 were considered significant.
Our data demonstrated a statistically significant lower CD8 count during the pretreatment stage (Mean 1337.30, SD 922.340) compared to the control group (Mean 1944.13, SD 948.931), p=0.003. No such differences in CD8% were observed between the two groups.
As shown in Table 1 there was a statistically significant higher CD8+CD38+% in the pretreatment stage (Mean 32.4514, SD 16.8007) compared to the control group (Mean 19.4628, SD 9.75555), p=0.000.
| T subsets | HBV patients (n=50) Before treatment | Healthy controls (n=40) | t-test | ||
|---|---|---|---|---|---|
| Mean | Std. deviation | Mean | Std. deviation | Sig. | |
| Lymphocytes% | 37.134 | 10.8678 | 36.276 | 8.896 | 0.688** |
| CD8% | 29.574 | 8.1069 | 30.469 | 9.8084 | 0.637** |
| CD8 count | 1337.3 | 922.34 | 1944.13 | 948.931 | 0.003* |
| CD38% | 32.4514 | 16.80076 | 19.4628 | 9.75555 | 0.000* |
| CD38 COUNT | 405.62 | 360.162 | 367.58 | 242.084 | 0.569** |
*Significant: <0.05
**Not Significant: >0.05
Table 1: Counts and proportions of peripheral lymphocyte subset in control group and patients in pretreatment stage.
After assessment we found that there was no statistically significant difference in CD8+ and CD8+CD38+ count during treatment follow up visits as shown in Table 2.
| I. Before treatment |
II. After 6 months |
III. After 12 months |
Sig. of difference (1 vs. 11) | Sig. of difference (1 vs. 11I) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Mean | Std. deviation | Mean | Std. deviation | |||
| CD8 count | 1337.3 | 922.34 | 1569.46 | 748.517 | 1359.44 | 724.362 | 0.148** | 0.888** |
| CD38 count | 405.62 | 360162 | 358.13 | 190.822 | 330.2 | 230.963 | 0.404** | 0.173** |
*Significant: <0.05
**Not Significant: >0.05
Table 2: Changes in the count of CD8 and CD38 during treatment follow up of patients.
There was a statistically significant lower CD8 count in visit II 6 months of after starting HBV therapy (Mean 1569.46, SD 748.517) compared to the control group (Mean 1944.13, SD 948.931), p= 0.039. Also there was a statistically significant increase in CD38% in visit II 6 months of after initiating HBV therapy (Mean 26.8182, SD 15.42536) compared to the control group (Mean 19.4628, SD 9.75555), p= 0.010.
In visit III we found that there was a highly significant decrease in CD8 count 12 months after of beginning HBV therapy (Mean 1359.44, SD 724.362) compared to the control group (Mean 1944.13, SD 948.931), p=0.001.
Our results demonstrated a statistically significant decrease in HBV DNA (log) in pretreatment stage visit I (Mean 4.4240, SD 1.58719) compared to visit II, after 6 months of starting HBV therapy (Mean 0.7381, SD 1.60791), p<0.001. Moreover, there was a statistically significant decrease in HBV DNA (log) in pretreatment stage visit I (Mean 4.4240, SD 1.58719) compared to visit III 12 months after initiating HBV therapy (Mean 0.2714, SD 0.84726), p<0.001 (Table 3).
| I. Before treatment |
II. After 6 months |
III. After 12 months |
Sig. of difference (1 vs.11) | Sig. of difference (1 vs.11I) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Std. deviation | Mean | Std. deviation | Mean | Std. deviation | |||
| CD8 count | 1337.3 | 922.34 | 1569.46 | 748.517 | 1359.44 | 724.362 | 0.148** | 0.888** |
| CD38 count | 405.62 | 360162 | 358.13 | 190.822 | 330.2 | 230.963 | 0.404** | 0.173** |
| HBV DNA (log) | 4.424 | 1.58719 | 0.7381 | 1.60791 | 0.2714 | 0.84726 | <0.001* | <0.001* |
| HBsAg quant. | 8179.94 | 10067.46 | 6692.66 | 7592.071 | 6051 | 6729.246 | 0.002* | 0.003* |
*Significance: <0.05
**not Significance: >0.05
Table 3: Changes in the count of CD8, CD38, HBV DNA, and HBsAg quantitation at treatme nt follow up visits.
A further correlation analysis showed no significant relationship between the CD8+CD38+% and serum HBV DNA; however, there was significant correlation between CD8+CD38+ count and serum HBV DNA (Figure 1).
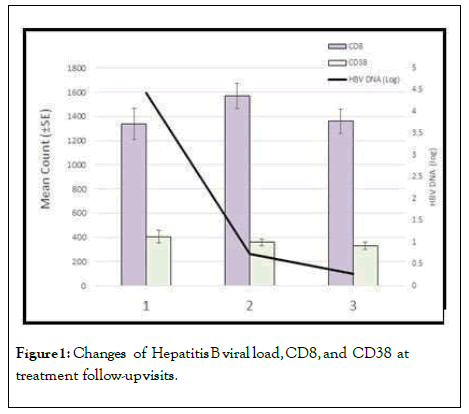
Figure 1: Changes of Hepatitis B viral load, CD8, and CD38 at treatment follow-up visits.
In our results, although there were no significant correlations between CD8+CD38+ and HBV DNA, a positive correlation was found between CD8+CD38+ and HBsAg quantitation (Figure 2).
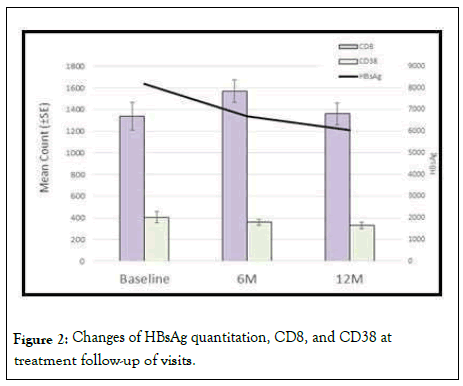
Figure 2: Changes of HBsAg quantitation, CD8, and CD38 at treatment follow-up of visits.
In addition to HBV DNA level and liver function, CHB is characterized by marked changes in lymphocyte subpopulations and their activation status, which hasonly been poorly described. In this study, we tried to give an initial assessment of the peripheral T lymphocyte subpopulations during HBV treatment.
Our data demonstrates that there is a statistically significant lower CD8 count during the pretreatment stage (Mean 1337.30, SD 922.340) compared to the control group (Mean 1944.13, SD 948.931), p=0.003, while no such difference in the CD8+CD38+ proportion was observed between the two groups.
Studies have revealed that T cellular immune responses are essential for disease pathogenesis [5], and have identified CD8+ T lymphocytes as the main cellular subset responsible for viral control [6].
As shown in Table 1 there was a high CD8+CD38+ proportion in the pretreatment stage (Mean 32.4514, SD 16.8007) compared to the control group (Mean 19.4628, SD 9.75555), p=0.000.
In a previous study Cao (2011) identified discoordinate T cell profiles in CHB patients, with decreased counts of CD8+ T cells and robust CD8+ T activation, determined by an increase in the proportions of CD8+CD38+ T cells [9].
After further assessment, there was no statistically significant difference in CD8+ and CD8+CD38+ count at treatment follow-up visits, as shown in Table 2. There was a statistically significant decrease in CD8 count in visit II, 6 months after beginning HBV therapy (Mean 1569.46, SD 748.517) compared to the control group (Mean 1944.13, SD 948.931), p=0.039. Furthermore there was a statistically significant increase in CD8+CD38+ proportion in visit II, 6 months after initiating HBV therapy (Mean 26.8182, SD 15.42536) compared to the control group (Mean 19.4628, SD 9.75555), p= 0.010, as shown in Table 2. In visit III we found that there was a highly significant decrease in CD8 count after 12 months after initiation of HBV therapy (Mean 1359.44, SD 724.362) when compared to the control group (Mean 1944.13, SD 948.931), p=0.001.
The peripheral CD8+CD38+ T cells in CHB patients showed a mode of persistent T cell activation, similar to what is observed in HIV infection. However, elevations of CD8+CD38+ T cells in CHB patients were not as high as those of observed in HIV, EBV or CMV infected patients [13,20]. This finding may be due to the relatively localized infection of HBV within liver tissue in contrast to systemic infections.
We also noticed that treatment resulted in the rapid decline of the CD8+CD38+% levels. This reduction in CD8+CD38+% began following the initial 6 months of treatment, and in most patients, CD8+CD38+% levels became fully normalized. This recovery of CD8+CD38+% confirmed the abnormal T cell activation which occurs as a result of the virological suppression failure in active HBV infection.
Our results demonstrate a statistically significant decrease in HBV DNA (log) in pretreatment stage visit I (Mean 4.4240, SD 1.58719) compared to visit II, 6 months after starting HBV therapy (Mean 0.7381, SD 1.60791), p<0.001. There was a statistically significant decrease in HBV DNA (log) in pretreatment stage visit I (Mean 4.4240, SD 1.58719) compared to visit III, 12 months after starting HBV therapy (Mean0.2714,SD 0.84726), p<0.001.
A further correlation analysis showed no significant relationship between CD8+CD38+% and serum HBV DNA, however there is significant correlation between CD8+CD38+ count and serum HBV DNA.
Previous research Cao showed a positive and significant relationship between the CD8+CD38+ T cell proportions and serum HBV DNA, which indicated a T phenotype drift with viral stimulation. However, at this stage, it remains unclear whether the observed immune activation was only a secondary change of viral insults, or part of HBV pathogenesis [9].
Studies of immune activation in HIV have suggested that CD8 T cell activation levels can predict the rate of disease progression independent of viral load, though the causes of this immune activation are likely multifactorial [21]. Furthermore, some have tried to target HIV- associated immune activation by using immunomodulatory agents in addition to antiretroviral therapy [22]. The role of immune activation in chronic HBV infection has been poorlydescribed.
Lino et al. performed a prospective investigation to clarify the applicability of flow cytometric enumeration of CD8+CD38+ T- cells in peripheral blood as a laboratorial indicator of CMV infection/reactivation in allogeneic hematopoietic stem cell (HSCT) recipients. The data presented in this study revealed that, the quantification of the CD8+CD38+ T cells is not useful in detecting infection/reactivation of cytomegalovirus in hematopoietic stem cell transplantation patients, as all patients exhibited high percentages of these cells regardless of the outcome of antigenemia. Therefore, the diagnosis and monitoring of CMV infection should be performed by established and reliable testing methods [23].
In our results a fact that drew attention was the lack of any significant correlation between CD8+CD38+ count and HBV DNA. However a positive correlation was found between CD8+CD38+ count and HBsAg quantitation.
HBsAg quantification was considered to be a promising, simple and inexpensive method to monitor viral replication in CHB patients treated with IFN. Studies have reported that the management of CHB can be optimized in daily practice using HBsAg quantification [17].
Indeed, availability of standardized commercial assays has renewed interest in quantitative serum HBsAg as a biomarker to stratify the risk of disease progression and predict treatment response, mainly in patients receiving PEG-IFN therapy. However, HBsAg quantification cannot replace viral load measurement in clinical practice. The combination of both titers has been shown to be useful for monitoring the natural history and outcome of the disease [24].
Our data revealed a significant correlation between HBV DNA and HBsAg quantitation at baseline. We noticed that the HBV DNA rapid decline is marked during the early phase of treatment compared to HBsAg quantitation. The combined use of HBsAg and HBV DNA assessment in patients with CHB can guide clinicians when evaluating the likelihood of treatment response and aid them when individualizing therapy strategies.
A longer follow up of patients after treatment may help to better explain the dynamics of CD8+CD38+ T cell subsets. Elevations of CD8+CD38+ T cells in chronic hepatitis B patients were not as high as those observed with other viruses, but this may be due to the relatively localized infection of HBV within liver tissue in contrast to systemic infections. The positive correlation between CD8+CD38+ T cells and HBsAg quantitation requires further evaluation. The combined use of CD8+CD38+ T cells, HBsAg quantitation and HBV DNA assessment in patients with CHB can guide clinicians as they guage the likelihood of treatment response. Outcome of HBV infection and the pathogenesis of liver disease are determined by immunemediated host-virus interaction, which is difficult to fully elucidate.
For prediction of response to oral antiviral therapy we recommend measurement of baseline HBsAg levels and follow up after 6 months and one year. Further studies characterizing patients with an observed cure (i.e., HBsAg loss) should also be conducted.
Citation: Shiha G, Toson EA, Elbeeh A, Abdellatif H ( 2020) Cellular Immune Response (CD8+ CD38+) in Relation to Hepatitis B Virus DNA Level and HBsAg Quantification in Hepatitis B Patients. J Antivir Antiretrovir. 12:204. DOI: 10.35-248/1948 5964.20.12.204
Received: 03-Aug-2020 Accepted: 17-Aug-2020 Published: 24-Aug-2020
Copyright: © 2020 Shiha G, et al. This is an open access article distributed under the terms of the Creative Commons Attributions License, which permits unrestricted use distribution, and reproduction in any medium, provided the orig inal author and source are credited.