Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Research Article - (2020)Volume 11, Issue 1
Poly (methyl methacrylate) was grafted onto cellulose acetate backbone using a “graft from” of Nitroxide Mediated Radical Polymerization (NMRP). The formation of cellulose acetate-co-poly (methyl methacrylate) using N-tert-butyl- N- (1-diethylphosphono-2,2-dimethylpropyl) (SG1)-nitroxide based macroalkoxyamine system was confirmed by FTIR and NMR analyses. The copolymer exhibited living characteristics as shown by NMR. DSC investigations showed a copolymer with a rich poly (methyl methacrylate) phase around 120°C and a rich cellulose acetate phase at around 175°C.
Cellulose acetate; Nitroxide mediated polymerization; N-tert-butyl-N- (1-diethylphosphono-2,2- dimethylpropyl) nitroxide (SG1); Poly (methyl methacrylate); Nitroxide mediated radical polymerization; Copolymer
Natural polymers, biopolymers and monomers from renewable resources [1-4] have been modified [5,6] by a variety of polymerization techniques. However, the chemical synthesis of biopolymers and modification of natural polymers are restrained due to their inherent nature. Copolymerization [7-9] of biomonomers and natural polymers with synthetic ones is one the most functional ways to modify natural polymers. Besides, the robust mechanical properties possessed by synthetic polymers are still desired in many applications. Furthermore, combining the properties of natural and synthetic polymers will produce a versatile material with even more excellent properties [10,11] as well as biodegradability [12-14]. Cellulose acetate which is obtained from the acetylation [15–17] of cellulose (the most abundant natural polymer on the surface of the earth) can boast of some excellent properties such as mechanical strength [18] compared to synthetic polymers. Other polysaccharides such as starch, chitosan, glucan and xanthan are also potential replacement to synthetic polymers because of the intrinsic properties [19] of the former which are comparable to those latter.
Nitroxide mediated radical polymerization (NMRP) amongst other modern controlled [20-23] radical polymerization techniques such as atom transfer radical polymerization (ATRP), ring opening polymerization (ROP) and reversible addition chain transfer polymerization (RAFT) has been extensively employed in the synthesis of biopolymers and synthetic polymers and the modification of natural polymers to obtained welldefined architectures [22,24–28]. NMRP stands because it is a facile metal free process and does not require any additional substances to initiate or perform polymerization. Chitosan [29] was heterogeneously modified with styrene and sodium 4- styrenesulfonate by Lefay et al. 2013 through NMRP. Poly (Nacryloyl- l-amino acid) was grafted on cellulose backbones through RAFT [30] polymerization by Liu et al. 2014. Polystyrene-graft-cellulose acetate was prepared in 2015 by Moreira et al. using controlled NMRP [7,31–33]. Cellulose and its derivatives have been grafted with polymethyl methacrylate through other polymerization techniques such as ATRP [34]. It should be also noted that there is no evidence supporting the complete elimination of the metal catalyst after the copolymer synthesis through ATRP. The presence of metal residues in the copolymer renders it unsuitable for bioapplications. However, the first synthesis of cellulose acetate-co-polymethyl methacrylate using so-called “ graft from ” [35-37] approach of nitroxide mediated radical polymerization to the best of my knowledge. The utilization of SG1 [37-41] nitroxde in the graft from NMRP, surmounts the difficulties due to steric hindrance encountered in modifying natural polymers and macromolecules through NMP.
Methyl methacrylate purchased from Sigma-Aldrich was purified by passing through aluminium oxide. 2M sodium hydroxide and hydrochloric acid and distilled water were purchased at Sigma- Aldrich. Bromoisobutyryl bromide (BIBB), triethylamine (TEA), dichloromethane (DCM), tetrahydrofuran (THF), acetonitrile (MeCN), dimethylformamide (DMF) were also purchased from Aldrich N-tert-butyl-N- (1-diethyl phosphono-2,2- dimethylpropyl) nitroxide (SG1) obtained from Arkema, France. Solvents such as hexane, ethyl acetate (EtOAc) and methanol (MeOH) were purified by simple distillation. THF and 1,4- Dioxane were refluxed over Na/benzophenone and freshly distilled before use. Dry DCM was freshly distilled over P2O5.
Cellulose acetate-bromide (CA-Br)
2 g of CA was dissolved in 10 ml of distilled THF in a 3-neck round bottom flask. The mixture was stirred until a homogenous solution was formed and then 5 ml of TEA (3.63 g, 35.85 mmol) was added. Argon was flushed into the flask to prevent moisture. Through an arm funnel 3 ml of bromoisobutyryl bromide (5.58 g, 24.27 mmol) was added dropwise at 0°C under stirring. The mixture was stirred at this temperature for 1 hr and then left at room temperature to react for 16 hrs. The crude product was centrifuge in order to separate the unwanted salt from the liquid product. The crude product was then precipitated in excess hexane to obtain pure white product which was dried on the rotavapor at 50°C.
Cellulose acetate-SG1 macroalkoxyamine (CA-SG1 MA)
1 g of CA-Br was dissolved in 10 ml of MeCN, then 0.946 g, 3.21 mmol of SG1 and 0.09 g, 0.32 mmol of Cu added. The mixture was deoxygenated for 20 min and then PMDETA was introduced into the reaction mixture. The mixture was allowed to react under argon at room temperature for about 16 hrs. The crude product was dissolved in ethyl acetate, washed with HCl and the volume was reduced by heating at 35°C on the rotavapor. The product was again dissolved in ethyl acetate and washed with NaHCO3 in order to remove HCl. The ethyl acetate was removed by means of a rotavapor and the final product (white solid) washed with water several times before drying on the rotavapor at 50°C.
Cellulose acetate-co-poly (methyl methacrylate)
100 mg of CA-SG1 macroalkoxyamine, 100 mg, 1 mmol of methyl methacrylate (MMA) were dissolved in 2 ml of 1,4- Dioxane in a vial. The mixture was deoxygenated for 20 min and heated at 95°C for over 16 hrs. The mixture was allowed to cool after 16 hrs and the crude product was precipitated in cold excess methanol. Another sample was synthesized under the same conditions and purified by precipitation in water to obtain the final product. Both final products were dried on the rotavapor at 50°C obtaining a yield of about 50%.
Fourier transform infra-red (FTIR) spectroscopy
In this work, all samples were analyzed by means of a 5.3.1 Perkin-Elmer Spectrum GX FT-IR System spectrophotometer in the range between 4000–5000 cm-1. Most of them were analyzed using the diffuse reflection method, mixing 1 mg of sample with 100 mg of an alkali halide, such as KBr, which does not contain bands in the mid-IR region of the spectrum. With this measurement method, background measurement is first performed on the KBr packed into the sample plate of the diffuse reflectance accessory. Next, the sample powder is diluted to 0.1% to 10% in the KBr powder and packed into the sample plate for infrared spectrum measurement. Samples dispersed in halide powder must be homogenously dispersed, with a particle size small enough not to cause scatter (theorectically <2 microns). Spectral analysis was performed in reflectance, with a spectral resolution of 4 cm-1 and 32 scans. Each spectrum was baseline corrected and normalized. To measure IR spectra of polymer solutions, they were pipetted into an IR cell with two NaCl windows (20×2 mm). Spectral analysis was performed in transmission mode with a spectral resolution of 4 cm-1 and 32 scans. The background spectrum was obtained using the two NaCl windows without sample solution.
Nuclear magnetic resonance (NMR) analysis
1H NMR spectra were recorded at 400 MHz with a Varian Mercury 400 spectrometer (chemical shifts are in part per million downfield from TMS); the solvents used were DMSO and CDCl3. All air-sensitive reactions were carried out in heatgun- dried glassware under an inert atmosphere of argon via standard Schlenk techniques.
Cellulose acetate-co-polymethyl methacrylate was synthesized in a three-step process according to Figure 1 above. An esterification was first of performed on cellulose acetate with bromoisobutyryl bromide thereby functionalizing the acetate groups. The second step is the formation of cellulose acetate macroalkoxyamine with SG1 nitroxide in a process of atom radical transfer addition (ATRA). And in the last step, polymethyl methacrylate was grafted from the macroalkoxyamine through NMP performed at 90°C to form the copolymer.
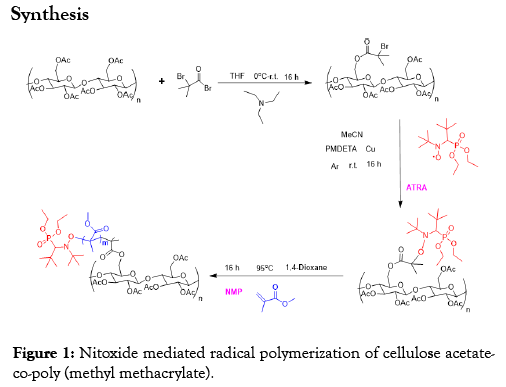
Figure 1: Nitoxide mediated radical polymerization of cellulose acetateco-poly (methyl methacrylate).
The peaks at 3487, 3026, 2886, 1369, 1236, 755 and 601 cm-1 are attributed to the OH groups of CA and those at 1748, 1433, 1371, and 1033 cm-1 show the presence of the acetyl group (Figure 2A). In Figure 2B, the band at 1748 cm-1 has intensified probably due to the presence of another C=O ester bond from reaction of cellulose with BIBB. Meanwhile, in CA-SG1 the emergence of the peak at 1054 cm-1 confirms the presence of SG1 (Figure 2C). The new peak at 1733 cm-1 can be attributed to the _COO- stretching vibration in PMMA and the characteristic peak for methyl methacrylate homopolymer at 1150 cm-1 from carbonyl groups confirming the CA-co-PMMA polymer (Figure 2D). These results are coherent with that in the literature [42–45].
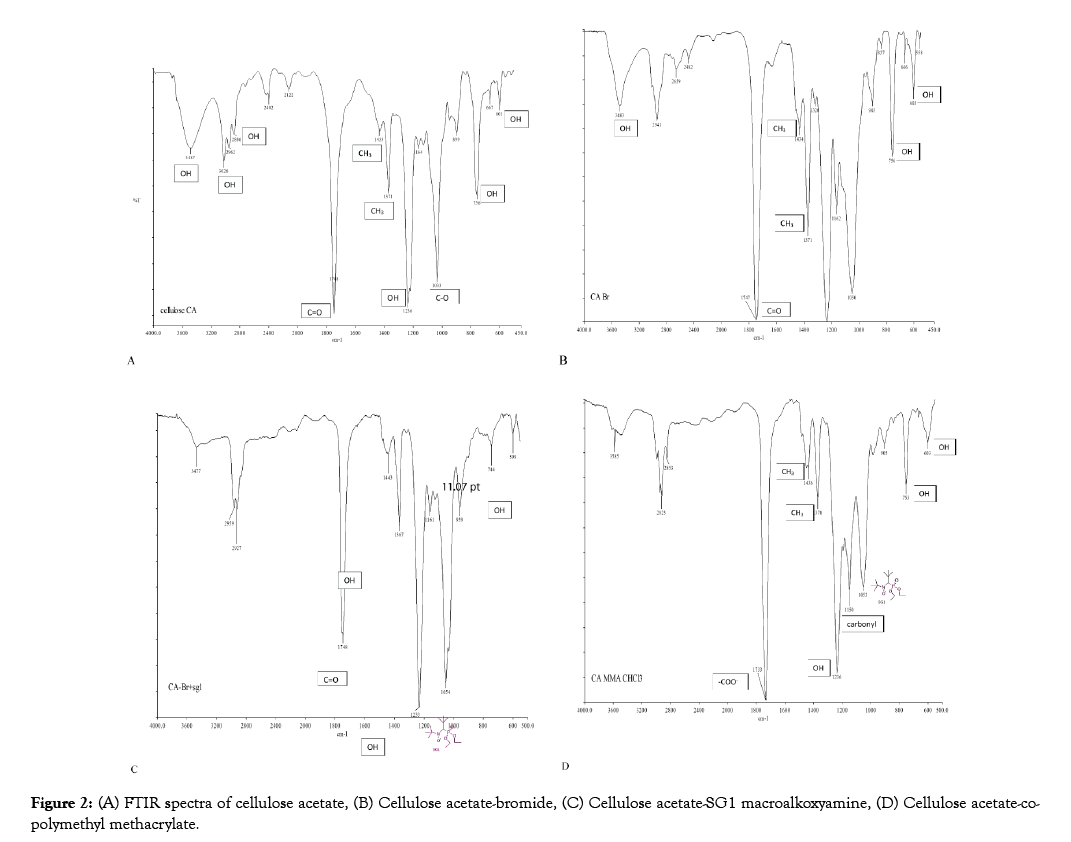
Figure 2: (A) FTIR spectra of cellulose acetate, (B) Cellulose acetate-bromide, (C) Cellulose acetate-SG1 macroalkoxyamine, (D) Cellulose acetate-copolymethyl methacrylate.
The peaks from 3.69 to 5.09 ppm in Figure 3A represent the hydroxyl groups of cellulose while acetyl groups are represented by the area around 2.0 ppm. The peak at 2.5 ppm is that of DMSO. The occurrence of a new peak at 1.46 ppm in Figure 3B which represents the bromide addition, in the formation of cellulose acetate-bromide while the peak at 7.5 ppm shows chloroform. t-butyl protons from SG1 made their appearance at around 1.0 ppm in Figure 3C, showing the formation of the cellulose acetate-SG1 macroalkoxyamine. In the cellulose-copolymethyl methacrylate polymer, the presence of polymethyl methacrylate is indicated by a new peak at 3.62 ppm, thereby confirming the formation of the copolymer in line with the literature [42]. The presence of t-butyl protons from SG1 in the copolymer indicates that it possesses a living character and further polymerization with other monomers is possible. The living character of polymers synthesized through NMP is a high ground for the formation of tailored polymer architectures.
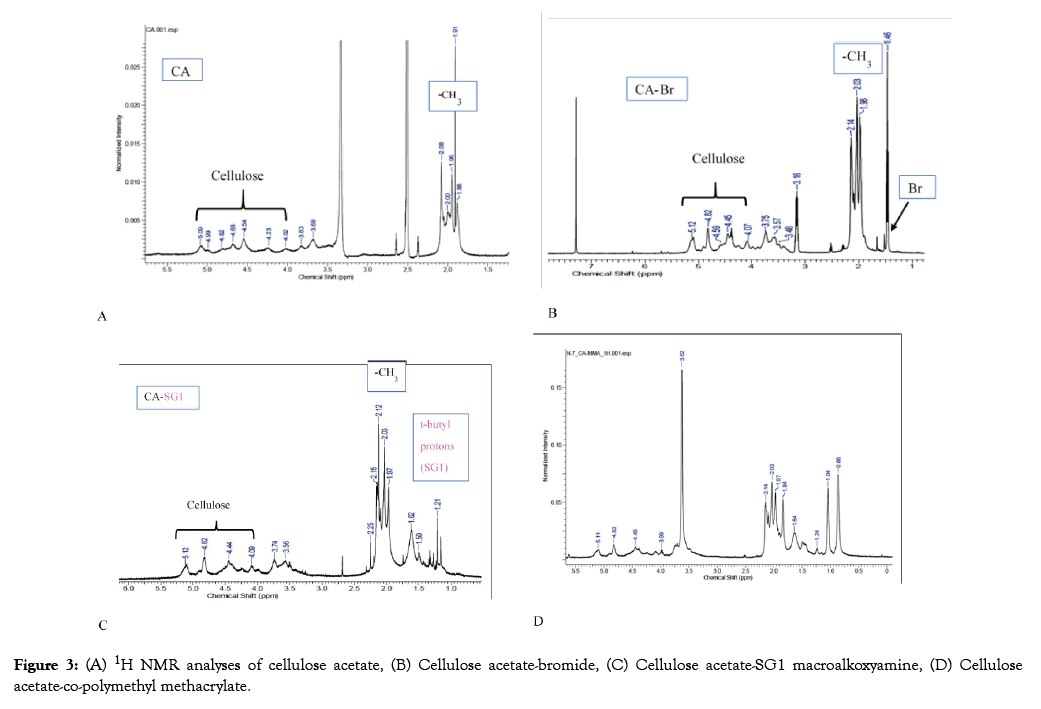
Figure 3: (A) 1H NMR analyses of cellulose acetate, (B) Cellulose acetate-bromide, (C) Cellulose acetate-SG1 macroalkoxyamine, (D) Cellulose acetate-co-polymethyl methacrylate.
DSC tests were performed from 50°C to 250°C with a scanning speed of 20°C/min. DSC results are in all cases compatible with the structure of "grafted copolymers". For both CA-PMMA samples, two glass transition temperatures (Tgs) for two separate phases were recorded; a phase rich in PMMA with Tg around 120°C and a rich CA phase with Tg around 175°C. Concerning these values, the Tg of the phase richer in PMMA is higher compared to that of the PMMA homopolymer. Furthermore, the Tg of the sample richer in CA is lower with respect to that of the pure CA homopolymer, also indicating a partial miscibility between the two polymers. The two samples showed in Figure 4 were obtained by precipitation using different solvents (methanol and water) but, interestingly, the same results were found. This confirms that, the crude cellulose acetate-copolymethyl methacrylate synthesized by the procedure described in this work can be purified by either precipitation in cold water or methanol [46-51].
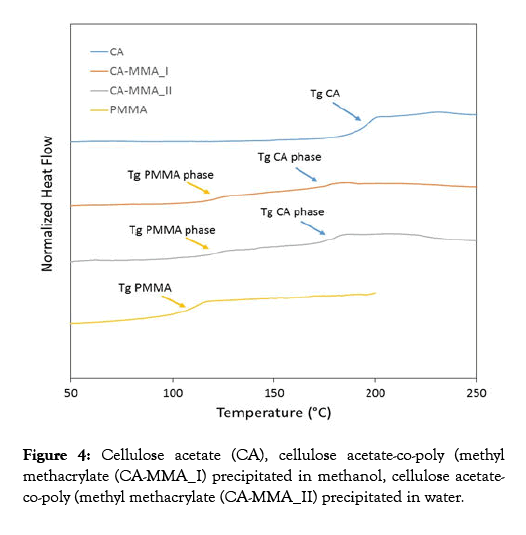
Figure 4: Cellulose acetate (CA), cellulose acetate-co-poly (methyl methacrylate (CA-MMA_I) precipitated in methanol, cellulose acetateco-poly (methyl methacrylate (CA-MMA_II) precipitated in water.
Polymethyl methacrylate was successfully grafted on cellulose acetate backbone through the “graft from” approach of nitroxide mediated polymerization confirmed by FTIR, 1H NMR and DSC. This is a very effective approach for copolymer synthesis. The graft from approach was facilitated by the formation of a macroalkoxyamine in a previous step using an atom transfer radical addition process. The copolymer possessed a living character as a result of the persistent radical effect and can act as a macroalkoxyamine, monomer and initiator in subsequent polymerization with other monomers. Moreover, its mechanical strength is portrayed by the high glass transition temperatures between the different phases. Since NMRP is one of the controlled polymerization techniques, the kinetics of will be studied in subsequent works.
Citation: Mbah VT, Celli A, Cipolletti R, Sabbatini S, Stipa P (2020) Cellulose Acetate-Graft-Poly (Methyl Methacrylate): A “Graft from” Approach of Nitroxide Mediated Radical Polymerization. J Chem Eng Process Technol 11:399. doi: 10.35248/2157-7048.20.11.399
Received: 12-Dec-2019 Accepted: 29-Jan-2020 Published: 07-Feb-2020 , DOI: 10.35248/2157-7048.20.11.399
Copyright: © 2020 Mbah VT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.