International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Review Article - (2023)Volume 11, Issue 4
There are several definitions for mTBI, which complicates diagnosis as well as our understanding of prognosis and optimal treatment. Here, we provide a comprehensive review of distinct perspectives on mTBI as well as a simplified overview of how these perspectives vary. This information should arm physicians with the pertinent information to effectively evaluate mTBI patients with multiple definitions for mTBI in mind.
Traumatic brain injury; Post-Concussive Syndrome; Post-traumatic amnesia; Glasgow Coma Scale
Traumatic Brain Injury (TBI), which disrupts brain function and can have fatal consequences, accounts for more than 2 million emergency department visits in the U.S. each year [1]. TBIs are classified as mild, moderate, or severe, and their severity has critical implications for both prognosis and treatment. Despite more than 50,000 annual U.S. deaths from TBI, concussionoften referred to as mild TBI (mTBI) is the most common form of TBI. Up to 80% of TBIs are mTBIs, on the other hand moderate TBIs and mTBIs together make up 90% of all TBI cases [1-3]. Unfortunately, because different organizations define TBIs differently, physicians have to deal with conflicting information that can impact their understanding of the patient’s prognosis and optimal treatment plan [4]. Part of the confusion around TBI diagnosis is that the inflammation and accompanying white matter degeneration that occur after TBI can continue for years [5]. Therefore, the direct effects of the TBI can be evaluated right after the injury, but it is difficult at this time point to also predict the long-term chemical and molecular changes. Without a better understanding of the ultimate impact of the injury on the brain, classifying TBI severity is challenging [6].
Here we discuss the lack of consensus on diagnostic criteria for classifying TBI severity as well as the inconsistent use of relevant diagnostic tools. We also highlight much of the resulting confusion, which can prevent physicians from distinguishing mild from moderate TBI and from accurately identifying Post-Concussive Syndrome (PCS), which, according to a 2019 workgroup at the International Congress of the Athlete Brain Health Foundation, should now be referred to as Persistent Symptoms after Concussion (PSaC) [7]. The condition may occur in more than 20% of mTBI cases. To combat this confusion, we lay out the different definitions and perspectives on mTBI to provide clinicians with a comprehensive view of mTBI and help guide their evaluation of patients whose injury may constitute a mild or a moderate TBI depending on the specific criteria that are relied upon for diagnosis [3,8-10].
Lack of consensus on diagnostic criteria obfuscates TBI severity
While TBI severity dictates the appropriate treatment modalities, the assigned severity does not provide clear and comprehensive information on the individual’s injury and thus does not on its own elucidate the best interventions [6]. Several organizations, including the Centers for Disease Control and Prevention (CDC), The World Health Organization (WHO), and The American Congress of Rehabilitation Medicine (ACRM) have attempted to formalize diagnostic criteria for mTBI, but lack of consensus around the definition of mTBI and the role of tools to evaluate TBI severity persist [11]. Definitions for mTBI overlap in that they all consider mTBI to be the result of an external force acting on the brain that causes a change in consciousness [11]. The American Congress of Rehabilitation Medicine in 1993, for instance, defined mTBI as occurring when a patient experiences a force applied to the brain and any one of the following; loss of consciousness, post-traumatic amnesia, focal neurological deficit, or any mental state alteration at the time of the trauma [3]. These criteria continue to be the most commonly used, though they are frequently criticized for their value in identifying mTBI.
Most organizations refer to cases where intracranial abnormalities or depressed skull fractures accompany a TBI that otherwise appear mild as ‘complicated mTBI’ [12]. However, others, such as the Department of Defense (DoD), categorize this latter type of injury as moderate TBI. Thus, those following definitions put forth by the DoD are likely to presume poorer prognoses in these cases than those following guidance from other organizations. It is unclear which organizations’ predictions are more accurate; thus, physicians should consider all possibilities when evaluating and treating patients with TBI.
Physicians should consider different TBI definitions when evaluating patients
Some researchers claim that there is little to no clinical meaningfulness of complicated versus uncomplicated mTBI, but there is also data suggesting that compared to uncomplicated mTBI, complicated mTBI is associated with worse quality of life and functional outcomes, including cognitive performance and time to return to work [12-15]. There is also ambiguity around how diagnostic criteria should be utilized in children versus adults. Some data suggests that identifying and understanding socalled complicated mTBI may be more important in children than in adults, as neuropsychological dysfunction is more likely when complicated mTBI occurs in children [16]. These results beg the question of whether what many refer to as complicated mTBI represent analogous pathologies in children versus adults or if they require distinct definitions and treatment management protocols.
These inconsistencies lead to debate amongst experts about how to classify TBIs and calls for revisiting these classification schemes [17]. It is important for physicians to recognize the disagreement amongst clinical organizations and understand the different perspectives for evaluating mTBI so that they can consider these issues when evaluating each patient. Rather than blindly following the definitions of a given organization, practitioners should be aware that there may be multiple possibilities for the severity of their patient’s mTBI and thus for their prognosis [18].
It is important to recognize that “mild” TBI often has significant long-term clinical implications
Several significant and sometimes long-term consequences are commonly observed in mTBI. For example, sleep disturbances, which have major health-related implications, occur in up to 80% of those who are told they suffered a simple concussion [18]. In addition, as with moderate and severe TBI, mTBI is associated with subsequent psychiatric and neurological illness [19]. There is no association between TBI severity and the occurrence of symptoms of depression one year later, highlighting important clinical similarities amongst all TBIs [20]. Even in cases of “mild” TBI, where there is no loss of consciousness, mTBI more than doubles the risk of dementia [21]. Given that substantial biochemical changes such as neuronal dysfunction and accompanying structural neuronal damage have been observed in mTBI, these clinically significant consequences should perhaps be unsurprising [22-24].
Inconsistent use of diagnostic tools requires physicians to use their expert judgment to evaluate TBI severity
When classifying the severity of TBI during the acute phase, a patient’s consciousness level is evaluated using the Glasgow Coma Scale (GCS) (Figure 1).

Figure 1: Glasgow Coma Scale. Adapted from Teasdale and Jennett, 1974.
GCS scores of 13 to 15 and 9 to 12 were once considered to indicate mild and moderate TBI, respectively. However, because this scale was originally intended to evaluate consciousness in patients who had not necessarily suffered TBI, it can lead to misclassification in TBI patients. To overcome the limitations of using only GCS to classify TBI severity, other factors are now also considered. However, how these other metrics are implemented to diagnose TBI varies, preventing a systematic way of classifying TBIs [25]. These factors include Computed Tomography (CT) results, the Abbreviated Injury Scale score, and durations of loss of consciousness and post-traumatic amnesia. Tools to diagnose PCS, where symptoms persist longer than expected following mTBI, also produce inconsistent results. For example, diagnostic criteria for PCS are different in the International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10) versus the Diagnostic and Statistical Manual-IV (DSM-IV), and PCS was not even included in the DSM-V [3,26]. These discrepancies require that physicians educate themselves on PCS to enhance their ability to accurately detect PCS in their mTBI patients [26,27].
Understanding risk factors can improve physicians’ ability to identify PCS
Fortunately, most people with mTBI or concussions recover fully and quickly. Nonetheless, a substantial portion of these patients, dubbed ‘the miserable minority’, suffer PCS [26,28,29]. The symptoms that persist in PCS include headaches, depression, anxiety, irritability, fatigue, dizziness, sleep disturbances, and cognitive difficulties [3,8,9]. Because these symptoms are nonspecific, PCS is difficult to diagnose, and its incidence may be underestimated [26].
There are some factors that increase the risk for PCS. For instance, those who suffered depression or anxiety before their injury have been shown to be more likely to suffer PCS 3 months following the injury than those without preexisting psychiatric conditions. While these pre-injury factors likely influence PCS risk, injury-specific and post-injury factors matter as well [26,30]. For example, compared to those with uncomplicated mTBI, those with complicated mTBI seem more likely to have PCS 3 months after their injury. In addition, those who experience acute stress following traumatic injuries are more likely than those without such stress to experience PCS [12].
Research that has focused on these differences has revealed that people with higher sensitivity to anxiety may perceive their injuries differently, which may contribute to PCS [31]. Thus, while PCS remains somewhat controversial, with some believing that neuroses may explain persistent symptoms, they appear to be physiological explanations for symptoms that last long after traumatic brain injury [9,32-41]. A comprehensive list of mTBI definitions according to the following organizations is provided in Figure 2.
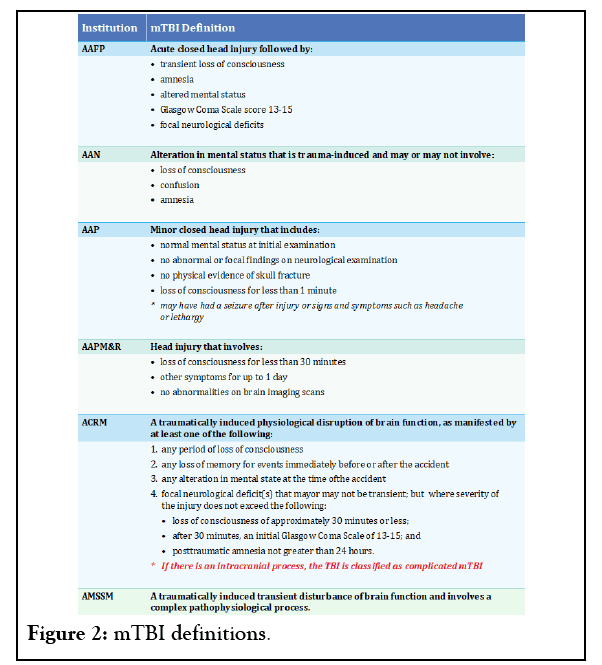
Figure 2: mTBI definitions.
• American Academy of Family Physicians (AAFP) [33]
• American Academy of Neurology (AAN) [34]
• American Academy of Pediatrics (AAP) [35]
• American Academy of Physical Medicine and Rehabilitation (AAPM&R) [36]
• American Congress of Rehabilitation Medicine (ACRM) [37]
• American Medical Society for Sports Medicine (AMSSM) [38]
• American Speech-Language-Hearing Association (ASHA) [39]
• Centers for Disease Control and Prevention (CDC) [40]
• Diagnostic and Statistical Manual-V (DSM-V) [41]
• U.S. Department of Veterans Affairs and U.S. Department of Defense (VA/DoD)
• World Health Organization (WHO) [35]
Though research has helped in improving our understanding of PCS, confusion around PCS continues. New calls to change the terminology to PSaC are accompanied by claims that this new terminology would reflect a paradigm shift in how all patients, including non-athletes, are treated when they experience symptoms beyond the typical time for which symptoms are expected in the context of concussion [7]. According to a working group that recently advocated for this change, a new term would help to focus patients and physicians on active rehabilitation in the group of patients who experience the persistent symptoms phenomenon [14].
Lack of consensus around how to define mTBI places an extra burden on physicians when evaluating mTBI patients and working to align expectations around prognosis and treatment duration. For example, the DoD classification of TBIs with GCS scores of 13 to 15 accompanied by an intracranial bleed as moderate is inconsistent with the commonly used requirement that GCS fall between 9 and 12 for a TBI to qualify as moderate. Nonetheless, it is unclear whose definitions of mild versus moderate TBI are more accurate. This confusion on how to determine mTBI severity is likely to be exacerbated as technology progresses because it will become easier to identify intracranial abnormalities and thus less clear how to incorporate those abnormalities into TBI assessment. Small abnormalities may be unrelated to the mTBI, leading to misdiagnoses. Physicians must therefore continue to educate themselves on the relevance of clinical findings to mTBI prognosis and the different perspectives on how to evaluate mTBI severity.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Lichtblau CH (2023) Challenges in the Classification and Understanding of Mild Traumatic Brain Injury. Int J Phys Med Rehabil. 11:668.
Received: 07-Feb-2023, Manuscript No. JPMR-23-21737; Editor assigned: 09-Apr-2023, Pre QC No. JPMR-23-21737 (PQ); Reviewed: 24-Feb-2023, QC No. JPMR-23-21737; Revised: 03-Mar-2023, Manuscript No. JPMR-23-21737 (R); Published: 10-Mar-2023 , DOI: 10.35248/2329-9096.23.11.668
Copyright: © 2023 Lichtblau CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.