Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2021)Volume 10, Issue 9
Background: Both denervation and hypertension can produce functional and structural damage in cavernous smooth muscle. Chaperone proteins, such as Heat Shock Proteins (HSPs), are expressed by cells under stress and prevent damage or reduce its magnitude. The purpose of this study is to determine the expression of HSP in cavernous smooth muscle of denervated and hypertensive rats.
Methods: HSP-70 and HSP-20 expression was analyzed in cavernous smooth muscle of 12 spontaneously hypertensive stroke prone (SHR-SP) male rats and 12 normotensive Wistar Kyoto rats. In addition, 24 Wistar rats, 12 week old, were divided in two groups, 16 of them received bilateral denervation and the rest were summited to a sham operation. Immunohistochemistry of the cavernous tissue was performed in all the samples.
Results: SHR-SP showed increased expression of HSP 20 (p=0.015) and HSP 70 (p=0.023) as compared with normotensive rats. Denervated rats showed decreased expression of HSP 20 (p=0.01) and HSP 70 (p=0.04), when compared with sham rats.
Conclusion: HSP-70 and HSP-20 show increased expression in spontaneously hypertensive rats compared with controls, probably because stress triggers protective mechanisms. On the other hand, bilaterally denervated rats show less HSP-70 and HSP-20 than sham animals, showing few chances for this tissue to regenerate after such a stress.
HSP 20; HSP 70; Hypoxia; Heat shock proteins, Experimental denervation
Experimental surgical denervation can reproduce the damage seen after a radical prostatectomy, a situation that causes cellular hypoxia [1,2]. Another clinical scenario where damage and remodeling of cavernous smooth muscle can be seen is arterial hypertension [3]. Different cellular processes may facilitate recovery after this damage. The response to stress of a cell is one of the most studied mechanisms, as it triggers several cytoprotective mechanisms, producing a change in the cellular machinery, a homeostatic reaction, which facilitates damage control and allows cellular repair [4]. Heat Shock Proteins, and its variants 20 KD (HSP-20) and 70 KD (HSP-70), are currently the best studied effectors of the cellular response to stress [5]. These proteins are rapidly synthesized and work like a “molecular chaperone” joining denatured proteins specifically. This union prevents the degenerative process to continue and helps to recover the protein regular shape repairing the damage. Their expression is increased in the presence of different cellular stress, such as the obstruction of the urinary bladder tissue hypoxia in transplanted kidneys or extracorporeal shockwave lithotripsy in rats [6-8].
The objective of this study was to determine the expression of chaperone proteins in damaged cavernosal smooth muscle caused either by denervation or arterial hypertension.
Twelve male 12-week-old, inbreed, spontaneously hypertensive stroke prone rats, (SHR-SP) (Group 1) were compared with twelve male normotensive Wistar Kyoto (WKY) rats of the same age (Group 2). In addition, twenty-four 12 week old, Wistar rats were divided in two groups, one with bilateral denervation (Group 3) (n=16) and the other with sham operation to serve as controls (Group4) (n=8).
Animals were housed in groups of four, with circadian light cycles, according to standard of care proposed by the University of Buenos Aires. They received water ad libitum and standard food (Cooperación-Argentina) throughout the experiment.
All animals were anesthetized using an intraperitoneal injection of ketamine 50 mg/kg and xylazine 6 mg/kg. A midline abdominal incision was made and the bladder and prostate were exposed. Extensive neurotomy of the pre-erectile periprostatic plexus was performed using a cautery on the lateral aspect of the prostate with no magnification, as previously described [9]. Neurotomy was performed bilaterally in group 3 and no neurotomy was performed in group 4 (sham group). The rats were treated with postoperative analgesia consisting of nalbuphine 1 mg/kg twice daily.
Rats from experiment 1 were sacrificed by the administration of a lethal dose of intraperitoneal sodium thiopental at the age 12 weeks. Rats from experiment 2 were sacrificed with the same procedure, twelve weeks after surgery. A midline abdominal incision was made and the penises were excised in bulk from the crus to the distal aspect.
Penile tissue was divided and the material was fixed in 10% formaldehyde with phosphate buffer (pH 7.2) to be included in paraffin. Three-micron cuts were performed and stained with Hematoxylin-Eosin (H and E), Periodic Acid-Schiff reagent (PAS) and Masson’s trichrome.
Immunolabeling of specimens was performed through a modified avidin-biotin-peroxidase complex technique. Following deparaffinization and rehydration, the sections were washed in Phosphate-Buffered Saline (PBS) for 5 minutes. Quenching of endogenous peroxidase activity was achieved by incubating the sections in 6% hydrogen peroxide in methanol for 60 minutes. After washing the sections in PBS, pH 7.2, for 20 minutes, the sections were incubated with blocking serum for 60 minutes. Thereafter, the sections were incubated with the primary antibody overnight, rinsed in PBS, and incubated with biotinylated secondary antibody for 30 minutes. After washing in PBS, the sections were incubated with streptavidin-peroxidase for 30 minutes. Then, the samples were processed by liquid DAB substrate pack concentrated (BioGenex, San Ramon, CA, USA) for 5 minutes. A mouse monoclonal Smooth Muscle α Actin (SMA) antibody (MU128-UC, BioGenex, San Ramon, CA, USA) was used to evaluate cavernosal smooth muscle status. In order to determine HSP-20 and HSP70 in cavernous tissue, a goat polyclonal IgG anti-HSP-20 (SC-1048) at a 1:100 dilution, and a goat polyclonal IgG anti-HSP-70 (SC-1060) at a 1:100 dilution were used respectively (Santa Cruz Biotechnology, Inc., CA, USA).
Between six and eight transverse histology sections from the penis of each animal were studied by an image analyzer (Image- Pro® Plus, version 4, Media-Cybernetics, Inc., Silver Spring, MD, USA). In order to compare similar segments of the corpus cavernosum of all rats, sections were taken from the proximal middle portion of the penis of each animal. Morphological analyses were performed with the observer blinded to the animal group, and the data were averaged. Cavernous tissue was delineated by the tunica albuginea, and then a quantification of the extent of immunostaining for the following parameters was performed: (a) cavernous smooth muscle layer in the cavernous space expressed as the percentage of a-SMA-positive immunostaining per area and (b) HSP20 expressed as a percentage of area, and (c) HSP70 expressed as a percentage of area. Negative controls (serial sections of each sample omitting the primary antibody) were also performed.
Values were expressed as mean ± SD. All statistical analyses were performed using absolute values and processed through Graph Pad-Prism, version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). For parameters with Gaussian distribution, comparisons among groups were carried out using t-test. For those parameters like histological data with non-Gaussian distribution, comparisons were performed by Mann–Whitney test. A p-value of <0.05 was considered significant.
SHR-SP presented an increased expression in HSP20, HSP70 when compared with WKY, as illustrated in Table 1 and Figure 1. On the other hand, the denervated rats of group 3 showed a decreased expression of HSP-20 and HSP-70 in cavernous muscles relative to sham animals, as displayed in Table 2 and Figure 2.
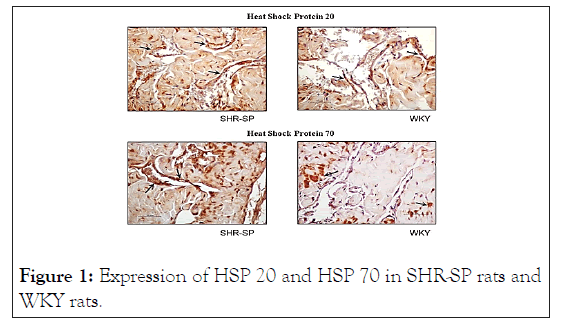
Figure 1: Expression of HSP 20 and HSP 70 in SHR-SP rats and WKY rats.
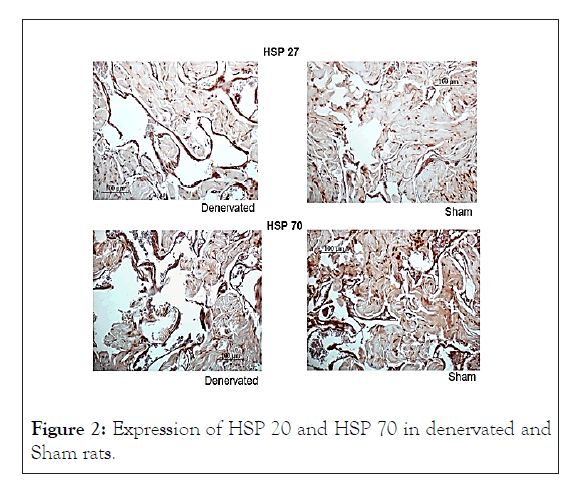
Figure 2: Expression of HSP 20 and HSP 70 in denervated and Sham rats.
| Mean ± SD % of positive staining/area | SHR-SP n=12 | WKY n=12 | p |
|---|---|---|---|
| HSP-20 | 9.9 ± 1.1 | 3.6 ± 0.7 | 0.015 |
| HSP-70 | 10.8 ± 1.3 | 3.1 ± 0.8 | 0.023 |
Table 1: Total expression of HSP20, HSP70 in SHR-SP and WKY.
| Mean ± DS % of positive staining/area | Denervated n=16 | Sham n=8 | p |
|---|---|---|---|
| HSP-20 | 1.63 ± 0.67 | 3.36 ± 0.68 | 0.01 |
| HSP-70 | 1.91 ± 0.85 | 2.98 ± 0.62 | 0.04 |
Table 2: Total expression of HSP-20 and HSP-70 in denervated
and sham rats.
In this study, we found that chaperone proteins have a higher total expression in cavernous smooth muscle from hypertensive animals compared with controls, probably because stress triggers protective mechanisms. On the other hand, denervated animals showed less total expression than sham animals. It is possible that the damage produced by bilateral cavernosal denervation, eliminates the chances of regeneration in this tissue.
Tissue hypoxia generates cellular death by necrosis and also induces apoptosis or programmed cell death. The HSP-70 diminishes apoptosis induced by ischemia protecting the cell thanks to different enzymatic mechanisms [4]. The HSP-20 has also been studied in the bladder smooth muscle of Sprague- Dawley rats. In such experiment, HSP-20 showed an increased expression in the presence of an obstruction in comparison to non-obstructed rats [10].
In addition, HSP expression is elevated in a wide range of human cancers and is implicated in tumor cell proliferation, differentiation, invasion, metastasis, death, and recognition by the immune system [11]. Broadly speaking, HSPs are considered protective molecules, which biologically and clinically could imply that the cells are being stressed, but that they have deployed a protective mechanism trending to prevent irreversible damage. Whilst HSPs expression can be interpreted as a marker of cell stress, it is not clear whether modifications of HSPs expression could affect the phenotype.
In an interesting article by Rodriguez-Iturbe, et al. the role of autoimmune reactivity induced by HSP 70 in the pathogenesis of essential hypertension is reviewed. Autoimmune reactivity directed against HSP70 may play a role in the pathogenesis of hypertension, as its expression is increased in the circulation and kidney of hypertensive patients, and genetic polymorphisms of this protein are associated with essential hypertension. Also, renal inflammation induced by immunity to HSP70 causes hypertension in laboratory animals, and administration of specific peptide sequences of HSP70 results in a protective antiinflammatory response that prevents and corrects salt-induced hypertension [12]. Relationship between HSPs and hypertension has also led to the investigation of its blood levels. A study was performed on 49 adults with an elevated waist circumference that were screened for arterial hypertension and other component disorders of the metabolic syndrome. HSPs concentrations in blood were significantly higher in the individuals with arterial hypertension than in their normotensive counterparts [13].
In the current study, chaperone proteins showed an increased expression in the hypertensive animal´s cavernous smooth muscle, compared with normotensive controls. This could suggest that although arterial hypertension generates some injury in the cavernous smooth muscle, it may also trigger protective mechanisms that might result in less damage of this affected tissue.
Experimental neuronal injury has been performed in different studies. Klass, et al. showed that chronic constriction of the sciatic nerve in rats instigates broad upregulation of small and large heat shock proteins in this tissue [14]. It should be noted that the damage produced by nerve contriction is less than that carried out in our experiment, which consisted in bilateral electroablation. In another study, Costigan, et al. demonstrated that HSP 27 is constitutively expressed at low levels in mediumsized lumbar dorsal root ganglion cells in adult rats. Transection of the sciatic nerve resulted in a nine-fold upregulation of HSP27 mRNA and protein in axotomized neurons in the ipsilateral Ganglion at 48 hr., while dorsal rhizotomy, injuring the central axon of this ganglion, did not upregulated HSP27 mRNA levels [15]. Strikingly, our results in denervated animals show downregulation rather than upregulation. One possible explanation is that when performing a bilateral denervation, the cavernous tissue suffers too extensive an effect for the HSPs to avoid its damage.
Experimental denervation, like the one we carry out in this work, has shown a functional correlate. Becher, et al. showed that administration of sildenafil helped to preserve cavernous smooth muscle integrity in denervated rats compared with placebo [9]. In the present study, denervated rats showed an absolute decrease in chaperone protein expression in the cavernous tissue. This finding may suggest that those cells, which suffered a lesion by bilateral denervation, were not able to develop a protective mechanism against the injury. Healthy cavernous smooth muscle cells are a key component to achieve a proper relaxation necessary to obtain a normal penile erection. An interesting clinical application of the findings of our work is that HSPs could be stimulated locally to reduce the damage caused by hypertension or denervation in erection. Voellmy, et al. published a review of the literature relating to localized heat activation of HSP promoters and HSP genes in the skin. They show that a multitude of different technologies has been explored in small animal models but only few publications examine HSP promoter activation in human skin [16]. For instance, the activation of HSP promoters in scalp, in particular in hair follicles, has been useful to prevent alopecia [17]. Considering that the cavernous tissue is relatively accessible, these applications could be used to promote the expression of HSP in this tissue subject to damage by hypertension or denervation
HSP-70 and HSP-20 show increased expression in spontaneously hypertensive rats compared with controls, probably because stress triggers protective mechanisms. On the other hand, bilaterally denervated rats show less HSP-70 and HSP-20 than sham animals, showing few chances for this tissue to regenerate after such a stress.
None
This paper received a Grant from the Latino American Society of Sexual Medicine (Sociedad Latinoamerica de Medicina Sexual SLAMS).
Citation: Sanguinetti H, Becher E, Mazza O (2021) Chaperone Proteins Expression in Cavernous Muscle of Denervated and Hypertensive Rats: Triggering Protective Mechanisms?. Andrology. 10:235.
Received: 23-Sep-2021 Accepted: 07-Oct-2021 Published: 14-Oct-2021 , DOI: 10.35248/2167-0250.21.10.235
Copyright: © 2021 Sanguinetti H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This paper received a Grant from the Latino American Society of Sexual Medicine (Sociedad Latinoamerica de Medicina Sexual SLAMS).