Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Research Article - (2020)Volume 10, Issue 1
Biological control; Postharvest disease management; Antagonism; Physiological variability; Stress tolerance
Aureobasidium pullulans (de Bary) Arnaud is a highly adaptable polymorphic species with ubiquitous presence in many diverse habitats [1]. It has been frequently isolated from various abiotic materials, soil and aqueous environments, animal substrates, as well as from plant surfaces in temperate and tropical habitats [2]. It was commonly isolated from variety of fruits including apples, pears, plums, nectarines, cherries, strawberries and grapes [3-13].
The fungus has great phenotypic plasticity and, depending on the environment, it may change growth from yeast to mycelial form as well as color and morphology of the colony [2,14-17]. Aureobasidium pullulans has utility in biotechnological applications as it produces an extracellular polysaccharide, pullulan, that is used in cosmetics, medicine and the food industry [18,19], and numerous enzymes, including amylases, lipases, and hemicellulose and xylan-degrading enzymes, that have a variety of industrial applications [2,15,20,21].
In the first half of the twentieth century, this fungus, under the old name Pullularia sp. was frequently isolated from decaying pears, grapes, and cherries during storage, especially when fruit was damaged by rain [22-24], from tomato where it was shown to cause fruit spots [25], and strawberries where it was one of the dominant fungi [26]. Plant pathogenicity has been attributed often to this fungus on several fruit crops although the evidence was based mostly on its association with the signs of a disease rather than causal agent and the effect. Furthermore, the later taxonomical data on Aureobasidium spp. disputes this and Zalar et al. stated that “It appears likely that the plant-invading, host- specific pathogens are consistently different from A. pullulans, which on host plants colonizes surfaces only, but unambiguously identified strains are needed to prove this” [17].
The complex taxonomy of A. pullulans was previously based on its morphology and nutritional physiology [27]. The species A. pullulans was redefined after multi loci DNA analysis using internal transcribed spacer (ITS), partial large subunit of 28S rDNA, partial intron and exon of genes coding β-tubulin (TUB), translation elongation factor (EF1α) and elongase (ELO) of a global collection of isolates from various habitats [17]. Four varieties were distinguished, two globally ubiquitous, i.e. var. pullulans and var. melanogenum, and var. subglaciale and var. namibiae, the last one with a single described strain. A majority of the var. pullulans strains are osmotolerant and come from plant habitats such as fruit surfaces, phyllosphere, tree slime efflux, but were also isolated from salterns. The taxonomic status of the A. pullulans species complex was finally resolved by genomic analysis uncovering large distances between the aligned genomes of different, above-mentioned, Aureobasidium pullulans varieties, together with substantial physiological differences, such as degree of melanisation, temperature growth range and NaCl tolerance. This was the basis for the redefinition of varieties into four well- defined species [2]. This is important from biotechnological, including biocontrol, perspectives as the emerging human pathogen now defined as a distinct species A. melanogenum is and no human pathogens are recognised among the other related species [2]. Furthermore, recent study of fifty A. pullulans genomes uncovered a homogeneous population structure with high levels of recombination, thus confirming that A. pullulans is a generalist without substantial specialization to its diverse habitats at the genomic level [28].
Antagonistic activity of A. pullulans (originally described as Pullularia pullulans) against several plant pathogens, including postharvest decay fungi such as Botrytis cinerea or Penicillium spp., was first described by Baigent and Ogawa [29] in a dual culture bioassay on potato-dextrose agar (PDA) medium. Ability of A. pullulans to control plant diseases was first demonstrated by Bhatt and Vaugham [30,31], who showed that spraying of strawberry plants at petal fall or early green fruit stage with a suspension of A. pullulans culture reduced gray mold fruit rot caused by B. cinerea by 31%. This strawberry isolate of A. pullulans (P. pullulans) was also able to stop growth of B. cinerea in dual culture, and its culture extract suppressed elongation of germ tube and mycelium growth of the pathogen.
The interest in using A. pullulans for biological control of plant diseases was invigorated by Falconi and Mendgen in early 1990’s [32,33] when searching for antagonists among naturally occurring microorganisms on apples against pathogens causing postharvest decays they found several very effective isolates of A. pullulans. Biocontrol potential, ecological fitness and plasticity of these isolates paved the way for the development of two commercial products based on a mixture of two isolates, DSM 14940 and DSM 14941 (original designation CF 10 and CF 40), “Blossom Protect” for the control of fire-blight of pome fruits caused by Erwinia amylovora and “Boni Protect” for the control of postharvest diseases of pome fruits [34-36].
These isolates were also effective in field application against gray mold of strawberries [37-39]. Effectiveness of a fruit isolate of A. pullulans (isolate L47) against gray mold of strawberries was earlier demonstrated by Lima et al. in laboratory and field studies [10,40,41]. Another isolate of A. pullulans, GRA1-2, isolated from strawberry fruit, greatly reduced gray mold of strawberries during development from green to red stage and on mature fruit after harvest [42]. Effectiveness of A. pullulans in controlling various fruit decays in postharvest applications was reported from several laboratories. Isolate L47 was effective against blue and gray mold of apple, green mold of grapefruit, and several diseases of tomato, whereas cherry isolates 533 and 547 were more effective in controlling postharvest decays of cherries and grape [43-46]. However, isolate L47 when combined with calcium chloride or sodium bicarbonate was very effective in pre- and postharvest applications in controlling postharvest rots of sweet cherries [44]. Fruit isolate, LS-30, was effective against major postharvest decays of apples when applied in combination with reduced doses of benomyl [9,47] and even lowered the ochratoxin A contamination in wine grapes [48,49].
Extensive studies with apple isolate Ach-1 [50] showed high effectiveness of this isolate in controlling blue mold of apples [51,52]. Peach isolates of A. pullulans, L1 and L8, were very effective in controlling brown rot caused by three Monilinia spp. on nectarines and peach [53,54], gray mold, blue mold and bitter root of apples [55], and stem end infection caused by B. cinerea on kiwifruit [56]. Aureobasidium pullulans was the most frequently isolated fungus from apples stored for six months in commercial cold chambers with strain ApB being the most potent inhibitor of B. cinerea [57]. Furthermore, A. pullulans was most frequently recovered from nectarine and plum fruit from early stage of fruit development to maturity and many of the isolates including PL7T2-S6I5, PL6T1-S2I11 and PL7T2-S2I5 showed high biocontrol activity against brown rot of stone caused by M. fructicola [7,8]. Recently, A. pullulans (isolate ST1-C9) and Metschnikowia pulcherrima (isolate FMB 24H-2), which are excellent colonizers of leaves and flowers, respectively, were used in combination to fill in the microbial void after UV-C/dark treatment of strawberry plants in the newly developed PhylloLux technology for controlling plant diseases [58,59].
It is undisputable that A. pullulans is a part of the resident microbial population of apple [4], and it would be surprising if an investigator isolating microbes from the surface of fruits mentioned above would not encounter isolates of A. pullulans. Looking at the many attributes of A. pullulans, it becomes axiomatic that this organism is becoming one of the most important biocontrol agents against postharvest diseases of various fruit crops. What makes strains of this fungus, often operating by different mechanisms of biocontrol, so effective in controlling various pre- and postharvest diseases and as attractive as a biocontrol agent for practical applications has not been fully explained. Thus, knowing common characteristics of the isolates with high biocontrol activity undoubtedly would help in selecting the best antagonists. The main objective of this study was to find common traits among various isolates with already determined biocontrol potential from different geographical locations that would be helpful in future selection of the best performing biocontrol isolates from the array of isolates of this vastly diverse species.
Strains
The strains of Aureobasidium pullulans were obtained from various laboratories, except two strains, EXF-11013 and EXF-11014, which were purchased and re-isolated from the commercial product Boni Protect® (BIO-FERM, Getzersdorf, Austria).
Most of the strains were isolated from fruits and some from leaves of fruit trees in different geographical locations including Europe, North America and Africa and were reported to control one or more postharvest disease of fruits (Table 1).
| Strain ID | Isolation source | Geographic origin | Date of isolation | Biocontrol potential against postharvest fruit decay pathogens |
|---|---|---|---|---|
| ST1-A24 | Apple wound in unmanaged orchard | Kearneysville, WV, USA | September, 1996 | On apples, pears, strawberries and stone fruits against Botrytis cinerea, Penicillum expansum, Colletotrichum acutatum, and Monilinia spp. |
| ST1-C9 | Apple wound in unmanaged orchard | Kearneysville, WV, USA | September, 1997 | On apples, pears, strawberries and stone fruits against: B. cinerea , P. expansum, C. acutatum, and Monilinia spp. |
| PL7T2-S2I5 (NRRL Y-62817) |
Carposphere of plum | Kearneysville, WV, USA | August, 2007 | On apples, pears, strawberries and stone fruits against B. cinerea , P. expansum, C. acutatum, and Monilinia spp. |
| PL6T1-S2I11 | Carposphere of plum | Kearneysville, WV, USA | August, 2008 | On apples, pears, strawberries and stone fruits against B. cinerea , P. expansum, C. acutatum, and Monilinia spp. |
| L1 | Carposphere of ‘Redhaven’ peaches | Romagna Region, Italy | July, 2012 | On stone fruits, apples or against B. cinerea , P. expansum, C. acutatum, and Monilinia spp. |
| L8 | Carposphere of ‘Redhaven’ peaches | Romagna Region, Italy | July, 2012 | On stone fruits and apples against B. cinerea , P. expansum, C. acutatum, and Monilinia spp. |
| LS30 (CBS 110902) | Carposphere of apple | Caserta, Campania Region, Italy | October, 1996 | On grapes and apples against P. expansum, B. cinerea , R. stolonifer and A. niger, and M. fructigena |
| AU1858 | Carposphere of apple | Campobasso, Molise Region, Italy | May, 2016 | On apples against B. cinerea and P. expansum |
| AU1869 | Carphosphere of cherry | Campobasso, Molise Region, Italy | June, 2016 | On apples against B. cinerea and P. expansum |
| AU1812 | Phyllosphere of walnut | Campobasso, Molise Region, Italy | July, 2016 | On apples against B. cinerea and P. expansum |
| Ach2-1 | Carposphere of apple | Gembloux, Belgium | 2004 | On apples against B. cinerea and P. expansum |
| Ach2-2 | Carposphere of apple | Gembloux, Belgium | 2004 | On apples against P. expansum |
| 1113-10 | Carposphere of apple | Meknes, Morocco |
2004 | On apples against B. cinerea and P. expansum |
| 1112-3 | Carposphere of apple | Meknes, Morocco | 2004 | On apples against P. expansum |
| AU1 (L47) (CBS 351.96) |
Table grapes | Rutigliano, Apulia Region, Italy | July, 1992 | On strawberries, grapes, sweet cherries, apples, tomatoes and grapefruit against B. cinerea , P. expansum, P. digitatum, R. stolonifer, M. laxa, and A. niger. |
| AU2 | Orange cv Navelate | Palagiano, Apulia Region, Italy | April, 2018 | Not tested |
| AU3 | Sweet cherries cv. Ferrovia | Conversano, Apulia Region, Italy | May, 2018 | Not tested |
| AU4 | Sweet cherries cv. Ferrovia | Conversano, Apulia Region, Italy | May, 2018 | Not tested |
| DMS 14940/DMS 14941 (EXF-11013) |
Phyllosphere of apple | Konstanz, Germany | June-October, 1989 | On apples against P. expansum and M. fructigena |
| DMS 14940/DMS 14941 (EXF-11014) |
Phyllosphere of apple | Konstanz, Germany | June-October, 1989 | On apples against P. expansum and M. fructigena |
Table 1: List of Aureobasidium pullulans strains used in this study.
The cultures were maintained on Malt Extract Agar (MEA) medium composed of 2% malt extract, 0.1% peptone (Conda, Pronadisa, Spain), 2% glucose (Kemika, Ovada, Italy) and 2% agar (Formedium, Hunstanton, United Kingdom), in deionized water.
DNA extraction and amplification
DNA extraction was performed from 7-day-old cultures according to Ende and de Hoog [60]. Aureobasidium isolates were characterised by sequencing the entire internal transcribed spacer (ITS) regions of ribosomal DNA by using primers ITS5/ITS4 [61] and D1 and D2 domains of the nuclear 28S ribosomal DNA (D1/D2) amplified using primers NL1/NL4 [62].
Sequences were generated through Sanger sequencing (16 ABI 3730×l) using a commercial provider (Microsynth, Wien, Austria). The sequence chromatograms were manually checked for quality using the FinchTV software (Geospiza, Los Altos, CA). The obtained sequences were deposited at the National Centre for Biotechnology Information (NCBI) under MN417119- MN417137 (D1/D2) and MN417030 - MN417048 (ITS).
Phylogenetic analysis
Sequences were checked against the NCBI database using the Basic Local Alignment Search Tool (BLASTn) [63]. Sequences of most closely related type strains and other related strains were selected and aligned using MUSCLE as implemented in MEGA, version 7.0 [64] and the alignments were manually adjusted.
The phylogenetic analysis was based on concatenated alignments of ITS and D1/D2 sequences of A. pullulans and other related species of this genus as well as fungi from other genera and was made with MEGA 7 [64] using the maximum-likelihood algorithm and Kimura 2-parameter evolutionary models. Evolutionary rates among sites were modelled using the Gamma distribution. Gaps were treated as missing data. Internal branch support was assessed based on 500 bootstrapped datasets. Selenophoma linicola, CBS 468.46 (T) was used as an out-group. The ITS and D1/D2 analyses involved nucleotide sequences of 27 strains. All positions containing gaps and missing data were eliminated. There were a total of 489 and 504 positions in the final datasets, in ITS and D1/D2 rDNA, respectively.
Heterotrophic and oligotrophic growth conditions
The growth of A. pullulans strains was assessed under heterotrophic conditions at 0°C, 24°C (optimal) and 37°C (human body temperature), and under oligotrophic conditions at 24°C and 37°C. Cell suspensions were prepared in deionized water from cultures grown on MEA medium and the concentrations were adjusted to OD600=0.5 in deionized water using a spectrophotometer (Shimadzu UV-1800, Shimadzu Corp, Reinach, Switzerland).
Five microliters of the cell suspensions were spotted on defined Yeast Nitrogen Base (YNB) medium (pH 7.0) composed of 0.17% (w/v) yeast nitrogen base (Qbiogene, Carlsbad, CA, USA), 0.5% ammonium sulphate (Sigma-Aldrich, St.Louis, MO, USA), 2% glucose (Kemika, Ovada, Italy), and 2% agar (Formedium) in deionized water. Similarly, the strains were inoculated on hundred-fold diluted YNB medium (diluted in deionized water) and pure 2% agar medium (Formedium).
In the case of filamentous strain L1, for which cell suspensions could not been done, the media were inoculated with 4 mm diameter plugs of the mycelium taken from the margin of an actively growing colony. Plates were incubated at 24°C for 14 days, at 37°C for 21 days and at 0°C for 30 days.
Biofilm quantification
The ability of biofilm formation was quantified using modified crystal violet assay described previously [65]. Briefly, cultures in the exponential growth phase in YNB medium were adjusted to OD590=0.15 in ten-fold diluted YNB. One hundred microliters of four parallel samples of the cell suspension were inoculated into a Nunc-TSP plate (Thermo Scientific, Waltham, MA, USA). After 48 h of stationary incubation at 24°C, 100 μl of 1% (w/v) crystal violet was added to each well.
The plates were incubated for 30 minutes then the wells were washed with sterile deionized water. Finally, the biofilms were dissolved in 100 μl of 10% (w/v) sodium dodecyl sulphate. The absorbance of the suspension was measured at 590 nm (A590) using CytationI3 Imaging Reader and Gen5 Microplate Reader and Imager Software (both BioTek Instruments, Inc., Winooski, VT, USA).
Each experiment was performed twice and the data were analysed using PAST 3.20 software [66]. The analysis of statistically significant differences among pairs of means of strains with the highest and the lowest production of biofilms was performed by Tukey’s pairwise test (In figure 1 marked with *, p<0.01).
Enzymatic activities
Activity of amylolytic, cellulolytic, xylanolytic, pectinolytic, esterolytic, proteolytic, ureolytic, chitinolytic, and β-glucosidase enzymes was determined on agar medium tests as described previously by Zajc et al. [67] briefly, cell suspensions of cultures grown on MEA medium were adjusted to OD600=1.0 in deionized water, and used for spot inoculations (5 μL) in triplicate onto the agar media containing the appropriate substrate. All of the assay plates were incubated at 24°C for 8 days and the activity of the enzymes was determined using either enzymatic index (EI) according to the Equation (1) or positive/negative reaction, depending on the enzyme:
EI = (2r of colony together with precipitation or clearing zone) × (2r of the colony)-1 (1)
The amylolytic activity was detected on a medium amended with soluble starch as previously described [67]. After the incubation period, assay plates were exposed to iodine vapour (Sigma-Aldrich, Saint Louis, MO, USA) and the clear zone around colonies indicating the enzyme activity was measured. The cellulolytic and xylanolytic activities were determined on a medium amended with carboxymethyl cellulose and xylan (CMC; Sigma-Aldrich), respectively, which after the incubation period were stained with Congo-Red (Merck, Darmstadt, Germany) and the clear zone around colonies indicating enzyme activity was measured [67].
The pectinase activity was determined on a medium amended with apple pectin (Sigma-Aldrich), which, after the incubation period, was flooded with iodine solution and the positive reactions defined as a clear zone around the colonies was measured [68-70]. The β-glucosidase activity was determined on a medium amended with aesculin (Sigma-Aldrich), which, after the incubation period, in positive reaction formed a black complex in the medium resulting from interaction between degradation products from aesculin (aesculetin) and ferric citrate [71].
The chitinase activity was determined on a medium amended with colloidal chitin prepared from crab shells (Sigma-Aldrich) which, after the incubation period, in positive reaction formed a purple zone around the colonies [67,72]. The caseinase activity was determined on a medium amended with casein which, after the incubation period, in positive reaction was defined as a clear zone around colonies [73,74].
The esterase activity was determined on a medium amended with Tween-80 and bromocresol purple which, after the incubation period, in positive reaction turned the medium purple with a white zone of precipitate around colonies [75]. The urease activity was determined on a medium amended with urea which, after the incubation period, in positive reaction turned medium from red to purple while the control plates (without urea) remained yellow [67,71].
Siderophore production
Production of siderophores was determined on a medium amended with chrome azurol S (CAS) in the presence of 1,4-piperazinediethanesulfonic acid (PIPES) (Sigma-Aldrich) as described previously [76,77]. Five microliters of aqueous suspension (OD600 =1.0 ) of the cells was placed in the middle of the agar plates and incubated at 24°C and 37°C for 14 and 21 days, respectively.
Osmotic tolerance
Tolerance to osmotic stress caused by NaCl was determined by growth of the strains on YNB agar plates amended with NaCl at concentrations ranging from 14% to 18% (w/v) in one percent increments. Plates were inoculated with 5 μl of aqueous cell suspensions of OD600 =0.5 serially diluted from 10-1 to 10-4 and incubated at 24°C for 21 days when the growth was determined.
High temperature tolerance
Tolerance of the strains to high temperature was determined by their survival after exposure to 50 °C for different periods of time. Cell suspensions of actively growing cultures in YNB medium were adjusted to OD600 =0.5 and 1 mL of each culture was incubated at 50°C for 2, 4, 6, and 24 h. At each time point 50 μl of the cell suspension was taken from the temperature-stressed cultures and serially ten-fold diluted (10-1 to 10-4 ) in deionized water. Five microliters of each diluted suspension was spotted on YNB agar plates. The plates were incubated at 24°C for 14 days when growth of the colonies was determined.
We studied physiological characteristics, such as growth at oligotrophic conditions, growth at low (0°C) and human body temperature (37°C), tolerance to high temperature (50°C) and salinity (up to 18 % NaCl, w/v) stress, biofilm forming capacity and production of siderophores and various enzymatic activities (amylolytic, cellulolytic, xylanolytic, pectinolytic, esterolytic, proteolytic, ureolytic, chitinolytic, β-glucosidase activity) of 20 strains of Aureobasidium pullulans most of them (17) with known biocontrol activity against various plant pathogens. Two strains were purchased from BIO-FERM (BoniProtect®), whereas the rest were obtained from various laboratories.
Heterotrophic and oligotrophic growth conditions
All the strains grew on oligotrophic media prepared as 100-fold diluted YNB medium and on water agar medium without any added carbon or nitrogen sources at 24°C (Table 2).
| Growth conditions | Time of incubation (days) | Temperature (°C) | Growth |
|---|---|---|---|
| Heterotrophic (YNB) | 30 | 0 | + |
| 14 | 24 | + | |
| 21 | 37 | − | |
| Oligotrophic (0.01 × YNB, pure agar) | 14 | 24 | + |
| 21 | 37 | − |
Table 2: Growth of the A. pullulans strains under heterotrophic conditions at 0°C, 24°C and 37°C on yeast nitrogen base (YNB) and under oligotrophic conditions at 24°C and 37°C on 100-times diluted YNB and pure agar medium.
All strains grew also at 0°C on the “full strength” defined YNB medium. Importantly, none of the tested strains grew at 37°C either on oligotrophic medium or on standard growth medium.
Biofilm formation
All A. pullulans strains produced biofilms based on biofilm-bound crystal violet absorbance at 590 nm (Figure 1).
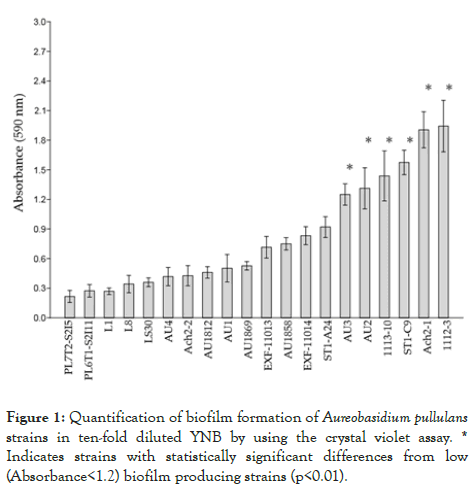
Figure 1: Quantification of biofilm formation of Aureobasidium pullulans strains in ten-fold diluted YNB by using the crystal violet assay. * Indicates strains with statistically significant differences from low (Absorbance<1.2) biofilm producing strains (p<0.01).
Some strains, such as Ach2-1 and 1112-3, showed pronounced biofilm production during the incubation period of the test. Pairwise comparisons of means of absorbance values among the strains according to Tukey’s test showed significant differences (p<0.01) between the strains with the highest (AU3, AU2, 1113- 10, ST1-C9, Ach2-1 and 1112-3) and the lowest (PL7T2-S2I5, PL6T1-S2I11, L1, L8, LS30, AU4, Ach2-2, AU1812, AU1 and AU1869) biofilm producing capacity.
Siderophore production
The majority of strains produced easily detectable siderophores on CAS agar at 24°C, with the exceptions of strains 1113-10, which had no noticeable halo around the colony, and AU1869, which could not grow on CAS agar (Figure 2).
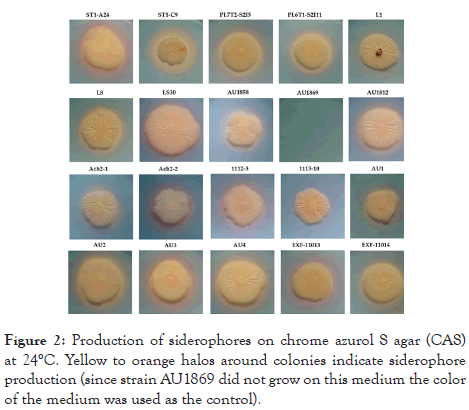
Figure 2: Production of siderophores on chrome azurol S agar (CAS) at 24°C. Yellow to orange halos around colonies indicate siderophore production (since strain AU1869 did not grow on this medium the color of the medium was used as the control).
The strain ST1-C9 was the most potent siderophore producer with the widest yellow halo indicating strong chelation of iron from the complexes in the CAS medium.
None of the strains either grew or produced siderophores at 37°C, even after prolonged incubation of 21 days (the incubation time at 24°C was 14 days).
Enzymatic activities
The strains included in this study were analysed for degradation of different substrates, related to plants (i.e., starch, cellulose, pectins, xylans, aesculin), fungi (chitin), animals (casein, urea) or other commonly encountered substrates (like fatty acids).
All the strains showed esterase, caseinase and β-glucosidase (on aesculin) activities (all strains had positive “+” reaction, not shown in Table 3) as well as amylase, cellulase and pectinase activities with varying enzymatic indices (Table 3).
Hierarchical clustering of the presence or absence of the enzymatic activities of the strains clearly shows the groups of strains with the same enzymatic profiles (Figure 3).
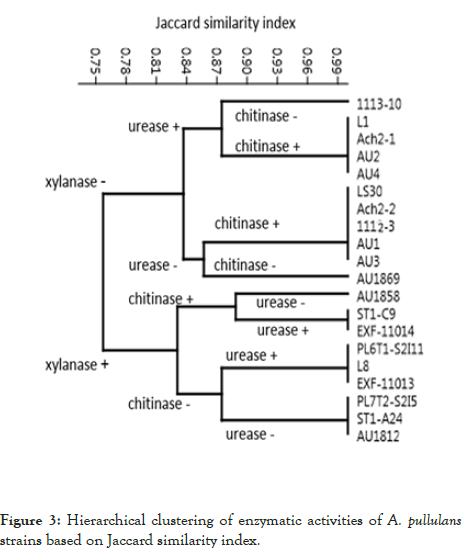
Figure 3: Hierarchical clustering of enzymatic activities of A. pullulans strains based on Jaccard similarity index.
The strains differed in exhibiting or lacking xylanase, chitinase and urease activities (Table 3 and Figure 3): two groups of strains were first segregated based on xylanase activity, then each group was further divided into four based on urease or chitinase activity to finally determine the strains composing one of the eight groups at the leaf nodes of the dendrogram.
| Strain | Amylase (EI) | Cellulase (EI) | Pectinase (EI) | Xylanase (EI) | Chitinase (±)* | Urease (±)* |
|---|---|---|---|---|---|---|
| ST1-A24 | 2.74 ± 0.42 | 2.76 ± 0.33 | 1.62 ± 0.14 | 1.24 ± 0.49 | − | − |
| ST1-C9 | 1.5 ± 0.53 | 1.81 ± 0.13 | 1.8 ± 0.21 | 1.43 ± 0.52 | + | + |
| PL7T2-S2I5 | 2.4 ± 0.16 | 1.96 ± 0.36 | 2.13 ± 0.56 | 1.32 ± 0.34 | − | − |
| PL6T1-S2I11 | 1.97 ± 0.33 | 1.82±0.15 | 1.65 ± 0.16 | 1.20 ± 0.16 | − | + |
| L1 | 1.3 ± 0.39 | 1.5 ± 0.32 | 1.45 ± 0.39 | − | + | + |
| L8 | 1.65 ± 0.22 | 1.61 ± 0.1 | 1.38 ± 0.37 | 1.36 ± 0.25 | − | + |
| LS30 | 3.13 ± 0.54 | 2.42 ± 0.24 | 1.65 ± 0.08 | − | + | − |
| AU1858 | 2.36 ± 0.54 | 1.82 ± 0.49 | 1.84 ± 0.18 | 1.60 ± 0.27 | + | − |
| AU1869 | 2.13 ± 0.23 | 2.95 ± 0.59 | 2.06 ± 0.2 | − | − | − |
| AU1812 | 2.11 ± 0.12 | 2.09 ± 0.11 | 1.66 ± 0.63 | 1.62 ± 0.40 | − | − |
| Ach2-1 | 1.88 ± 0.09 | 2.06 ± 0.16 | 1.32 ± 0.23 | − | + | + |
| Ach2-2 | 1.74 ± 0.14 | 1.86 ± 0.38 | 1.31 ± 0.25 | − | + | − |
| 1112-3 | 1.45 ± 0.33 | 2.35 ± 0.49 | 1.33 ± 0.45 | − | + | − |
| 1113-10 | 1.84 ± 0.15 | 1.95 ± 0.2 | 1.4 ± 0.24 | − | − | + |
| AU1 | 1.79 ± 0.2 | 2.04 ± 0.3 | 1.36 ± 0.36 | − | + | − |
| AU2 | 2.39 ± 0.28 | 2.03 ± 0.17 | 1.43 ± 0.33 | − | + | + |
| AU3 | 2.15 ± 0.41 | 2.02 ± 0.7 | 1.26 ± 0.22 | − | + | − |
| AU4 | 2.64 ± 0.09 | 2.27 ± 0.62 | 1.23 ± 0.27 | − | + | + |
| EXF-11013 | 1.63 ± 0.06 | 1.92 ± 0.25 | 1.34 ± 0.27 | 1.46 ± 0.32 | − | + |
| EXF-11014 | 1.48 ± 0.2 | 1.4 ± 0.09 | 1.04 ± 0.13 | 1.40 ± 0.27 | + | + |
* + indicates positive reaction; − Indicates negative reaction
Table 3: Enzymatic activities of the A. pullulans strains.
Salt tolerance
All the strains grew on defined YNB medium supplemented with 14% NaCl (Figure 4). However, at higher concentrations, the strains differ greatly in halotolerance. Eighteen strains grew on 15% NaCl, 17 strains on 16% NaCl, 15 strains on 17 % NaCl and 14 strains grew on medium with 18% NaCl, the highest concentration used in this study (Figure 4).
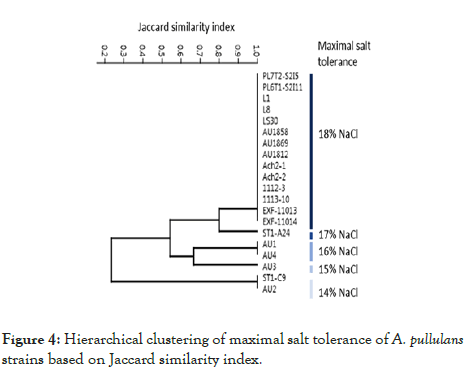
Figure 4: Hierarchical clustering of maximal salt tolerance of A. pullulans strains based on Jaccard similarity index.
Temperature tolerance
Strains differ in their tolerance to 50°C. As expected, the longer incubation time reduced the cell survival in the test tubes to final death. Two strains, PL7T2-S2I5 and EXF-Ach-2, did not survive 2 h exposure while the other 18 strains did. Only ten strains survived exposure for 4 h and four strains for 6 h. After 24 hours of exposure to 50°C only L1 and L8 strains could re-establish their growth (Figure 5)
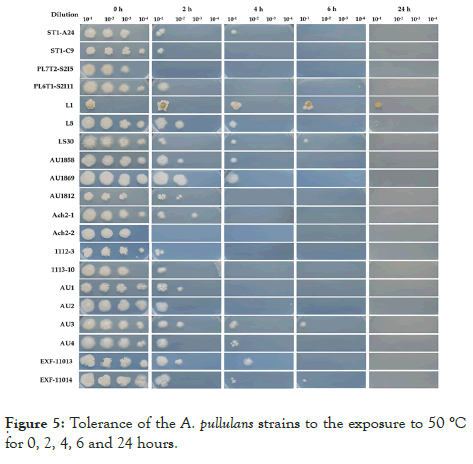
Figure 5: Tolerance of the A. pullulans strains to the exposure to 50 °C for 0, 2, 4, 6 and 24 hours.
Phylogenetic analysis
The phylogenetic tree constructed using the concatenated alignment of internal transcribed spacer (ITS) and the D1/D2 domains of the large subunit of the nuclear ribosomal RNA (rRNA) gene complex (LSU) regions confirmed that all the strains used in this study are most closely related to the ex-neotype strain A. pullulans (CBS 584.75) (Figure 6).
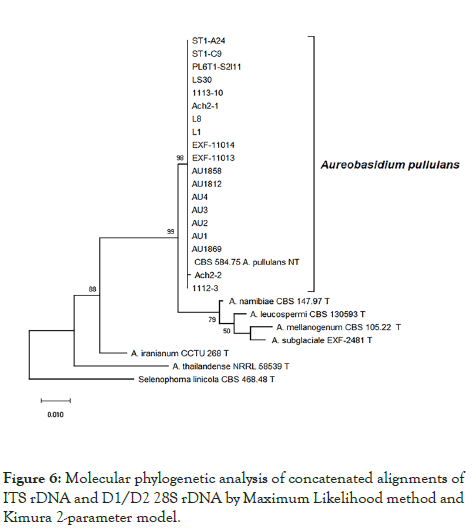
Figure 6: Molecular phylogenetic analysis of concatenated alignments of ITS rDNA and D1/D2 28S rDNA by Maximum Likelihood method and Kimura 2-parameter model.
The cosmopolitan ecology of Aureobasidium pullulans (Ascomycota, Dothideomycetes, Dothideales) implies its generalist behaviour. Strains of this fungus were isolated from plant surfaces [78,79], from indoor habitats [80-82] as well as from various more extreme environments like frozen, salt-preserved and dried food [83,84], hypersaline waters [85,86], glacial ice [17,87], aviation fuel tanks [88], and synthetic polymer surface [89]. The ability to thrive in these distinct habitats was linked to its great adaptability, morphological plasticity, nutritional versatility and rich array of secreted proteins [2], as well as production of antimicrobials and siderophores, and finally biofilm forming capacity [20,90].
All these characteristics in combination contribute to A. pullulans great capacity to outcompete other organisms in its environment either by competition for nutrients and space, and/or antibiosis via secretion of numerous compounds (e.g., siderophores, antibiotics, toxins, etc.) and even direct parasitism via employing lytic enzymes [54,91]. By employing several of these biocontrol mechanisms, it is not surprising that A. pullulans is one of the most promising biocontrol agents against a variety of phytopathogenic fungi and numerous research is dedicated to harnessing its vast potential [8,34,40,41].
Evidence presented in many publications from various research groups over the last few decades suggest differences in the degree of antagonistic potential and various prevailing mechanisms of biocontrol among individual strains of A. pullulans [52,55,57]. Thus, in the current study we aimed to describe some important characteristics of strains isolated in different parts of the world that might be important for the biological control of fruit pathogens and determine their usefulness for potential commercialization [59,92].
Various aspects of the polyextremotolerance of A. pullulans have been described, including tolerance to low temperatures, pH, salt and lack of nutrients [2]. However, none of the past studies systematically investigated the growth limits under extreme conditions. Here we show for the first time that A. pullulans is able to actively grow at 0°C.
This is a very important biocontrol trait because many fruits, especially those from a temperate climate, are stored at temperatures close to 0°C or even slightly below, as in the case of long-term storage of pears [12]. The temperature regimes on leaf and fruit surfaces in the field, and in high/low tunnels or greenhouses can vary greatly. Therefore, the ability to survive temperature fluctuation stress is a desirable trait for biocontrol agents. The strains included in this study have shown substantial tolerance to exposure to 50°C, the majority of strains survived 2 h exposure.
Even though most of the strains were able to survive high temperature stress, their growth was completely inhibited at 37°C. This eliminates the human pathogenicity concerns of A. pullulans as the ability of a fungus to thrive at 37°C and above (i.e., thermotolerance) is the most obvious virulence factor of fungal human pathogens and the most important risk factor when assessing the safety of potential biocontrol agents [57,67,93].
In addition to the inability to grow at 37°C, phylogenetic analysis based on internal transcribed spacer (ITS) and D1 and D2 domains near the 5' end of the 28S ribosomal RNA gene (large subunit rRNA) regions showed that all strains that we investigated are tightly related to the type species of A. pullulans (99 to 100% identity in general BLASTn searches).
This excludes the risk of using the emerging human pathogen A. melanogenum in biocontrol, a species that was until recently considered as a variety of A. pullulans. This removes the danger of abandoning the use of A. pullulans despite the huge investments that various laboratories have made in studying this fungus as an antagonist against various postharvest fruit pathogens and ensures us that the species will not share the fate of another well know antagonist with extraordinary versatility, Pseudomonas cepacia (now Burkholderia cepacia), which had to be abandoned due to the possibility of being an opportunistic human pathogen [94].
Osmotolerance, tested as a tolerance to NaCl, is known for A. pullulans from previous studies [17,85]. Confirming these results we noticed here that all strains grew on medium with 14% NaCl. Surprisingly, more than half of the tested strains (14) grew even with 18% NaCl, which is the highest concentration of NaCl so far reported for this species. Tolerance to elevated salinity enables compatibility with postharvest practices. For example, to minimize fruit injury during postharvest handling, field bins containing pears are submerged in a salt solution of sufficient specific gravity to allow pears to float [95]. Furthermore, tolerance to high osmolarity may also be related to adaptation to other stress conditions, such as oxidative, heavy metal, and heat and cold stress [92,96,97] some of which may play a significant role in biocontrol.
The ability to grow under oligotrophic conditions is another advantage when considering competition with microorganisms on plant surfaces. We found that all strains of A. pullulans that we tested are oligotrophic and were able to grow even on 100- fold diluted defined YNB medium and on pure agar without any carbon or nitrogen sources added. This is certainly an advantageous trait in biocontrol having in mind competition with other (pathogenic) species and cultivating the large quantities of biomass for commercial purposes.
Microbes overcome low availability of nutrients or even starvation by employing various strategies including the production of high- affinity iron-chelating compounds such as siderophores under iron-limiting conditions [98]. Siderophores have several different roles in virulence, fungal-host interactions, and resistance to oxidative stress [99]. Iron sequestration also has been involved in control of postharvest decays of fruits by A. pullulans [57] as well as in a few very effective yeast biocontrol agents such as Rhodotorula glutinis or Metschnikowia pulcherrima [100-102]. The radial growth of B. cinerea was 50% inhibited in dual culture with A. pullulans in the absence of iron, and there was no inhibition in media supplemented with ferric chloride, suggesting the role of iron sequestration in pathogen growth inhibition. However, this was not the case in the same test performed with P. expansum, that is less sensitive to iron deprivation [57]. In the present study we learned that only two strains were lacking the ability to produce siderophores under the test conditions, whereas the rest produced siderophores noticeable as yellow and purple colour changes of the otherwise blue CAS media. The two different colours of the halos suggests the production of two different types of siderophores, namely hydroxamate (yellow to orange colour change) and catechol-types (purple colour change) depending on their chemistry of the coordination sites with iron [103]. Production of hydroxamate siderophores can be found mainly in fungi, whereas interestingly, catecholates are primarily found in bacteria [104]. In the present study, we thus detected an alternative type of siderophores by A. pullulans, possibly fungal catecholates, but further characterization is needed to confirm this.
Adhesion and formation of biofilm is crucial for colonization of any surface including plant surfaces like leaves, roots or fruits [14]. Established mature biofilms increase resilience of the consortia of microorganisms to numerous abiotic and biotic stresses [105,106]. In light of biocontrol, this brings several benefits such as the spread of biocontrol agents throughout the plant surfaces in the form of a biofilm, which prevents the colonization by other, possibly plant pathogenic, microorganisms, and by niche exclusion due to intensive competition for space.
It may also limit access to nutrients and increase resistance to abiotic stresses [107]. Our study showed that all the A. pullulans strains produced biofilms; however, the strains differed greatly in the extent of production. This is in agreement with previous reports on the ability of A. pullulans to form biofilms on plant surfaces [90,108]. However, biofilm formation seems not to be essential for biocontrol activity considering the fact that 17 of the tested strains have proven strong antagonistic activities against plant pathogens but showed great variability in biofilm forming capacity.
Nevertheless, it was recently shown that biofilm-forming lifestyle improves the biocontrol activity of A. pullulans against the causal agent of sour rot in citrus, Geotrichum citri-aurantii , not only by niche exclusion but also via antibiosis by deforming the hyphae of the pathogen [90]. Since the addition of ammonium sulfate stimulated production of this biofilm, increased biocontrol activity and promoted survival of A. pullulans, it would be prudent to determine if such chemical supplement could improve the biocontrol activity of low biofilm producing strains observed in our study.
The production of a rich repertoire of extracellular enzymes enables not only good survival but also increases the competitive ability of antagonists, especially for nutrients, over fast growing, namely r-strategist microorganisms [109,110]. Degradation of plant biomass to obtain nutrients is a challenging task and requires combinations of various carbohydrate-active enzymes (CAZys), such as cellulases, β-glucosidases, xylanases, amylases, pectinase and numerous others.
Genome analysis of A. pullulans uncovered a plethora of plant biomass degrading enzymes comparable in richness to some plant pathogens [2] even though this species is known not to cause any plant diseases. In fact, A. pullulans together with Metschnikowia pulcherrima , had the highest production of extracellular enzymes such as proteases, β-glucosidases, lipases and polygalacturonases, among yeasts isolated from leaves, blossoms and fruit of temperate fruit trees [111]. Many strains from both of these yeast species are excellent biocontrol agents.
Enzymes directly effecting pathogens such as chitinases, caseinases, glucanases, and cellulases are also important in biocontrol through their direct antifungal activities [57,112] or by releasing oligomers capable of eliciting plant defensive responses [112-114]. However, under certain limiting growth conditions, like low or high temperatures, the competition for nutrients and space might not play the central role in biocontrol. This was demonstrated in a study that showed that at low temperatures (5°C) the production of antifungal enzymes, such as chitinases and β-1,3-glucanase of A. pullulans was delayed and therefore not involved in the initial mechanism of action against P. expansum [57].
Here we demonstrated that all strains produced pectinase, cellulase, amylase, β-glucosidase, caseinase, and esterase, and about half of the strains produced chitinase, xylanase and urease. Our results are in accordance with previously reported enzymatic activities of selected strains of A. pullulans [57,67,112] and uncovered the diversity among enzymatic activities of strains with reported biocontrol activities. The very rich secretome of all our A. pullulans strains enable them to be excellent plant colonizers and biocontrol agents against various fruit pathogens.
Our study, supported by numerous previous researches, suggests that there is no universal mechanism of action of biocontrol activity of A. pullulans. The outcome of competition of pathogens and a biocontrol agent, such as A. pullulans, seems to be based on a rather delicate balance of a plethora of characteristics of all three, biocontrol agent, its counteracting rival(s), and certain environmental factors [115].
The yeast-like fungus, A. pullulans, is a cosmopolitan generalist found on a wide range of plants. Seventeen strains used in this study were previously reported to have strong biocontrol activity against various pathogens causing postharvest fruit decays. Some strains are currently available as commercial products and it is very likely that more strains will be commercialized in the future. Diversity among the strains is thought to be based mainly on the different biocontrol mechanism(s) by which they operate and our results support this assertion.
However, we also showed certain commonalities among biocontrol strains related mainly to enzymes involved in the utilization of plant material and high tolerance to environmental stresses that may be helpful in searching for new biocontrol strains. Thus, “robust prediction of secretome proteins” together with tolerance to environmental stresses and the inability to grow at human body temperature may be useful as initial steps in evaluating a large number of new strains of A. pullulans for their potential usefulness for biological control of fruit decays before embarking on time consuming and costly fruit tests.
This study was supported by the state budget of the Slovenian Research Agency (Postdoctoral Project Z7-7436 to J. Zajc; Research Programmes P1-0170 and P1-0207; and Infrastructural Centre Mycosmo, MRIC UL). The authors declare no conflict of interest.
Citation: Zajc J, Cernosa A, Francesco AJ, Castoria R, Curtis FD, Lima G, et al. (2020) Characterization of Aureobasidium pullulans Isolates Selected as Biocontrol Agents Against Fruit Decay Pathogens. Fungal Genom Biol. 10:161.
Received: 20-Dec-2019 Accepted: 15-Jan-2020 Published: 22-Jan-2020 , DOI: 10.35248/2165-8056.20.10.161
Copyright: © 2020 Zajc J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.