Journal of Aeronautics & Aerospace Engineering
Open Access
ISSN: 2168-9792
ISSN: 2168-9792
Research Article - (2015) Volume 4, Issue 1
Aluminium carbide (Al4C3) has gained extensive attention due to its abrasive and creep resistance properties. Aim of the present study was to evaluate the impact of biofield treatment on physical and structural properties of Al4C3 powder. The Al4C3 powder was divided into two parts i.e. control and treated. Control part was remained as untreated and treated part received biofield treatment. Subsequently, control and treated Al4C3 samples were characterized using X-ray diffraction (XRD), surface area analyser and Fourier transform infrared spectroscopy (FTIR). XRD data revealed that lattice parameter and unit cell volume of treated Al4C3 samples were increased by 0.33 and 0.66% respectively, as compared to control. The density of treated Al4C3 samples was reduced upto 0.65% as compared to control. In addition, the molecular weight and crystallite size of treated Al4C3 samples were increased upto 0.66 and 249.53% respectively as compared to control. Furthermore, surface area of treated Al4C3 sample was increased by 5% as compared to control. The FT-IR spectra revealed no significant change in absorption peaks of treated Al4C3 samples as compared to control. Thus, XRD and surface area results suggest that biofield treatment has substantially altered the physical and structural properties of treated Al4C3 powder
<Keywords: Biofield treatment; Aluminium carbide powder; X-ray diffraction; Fourier transform infrared spectroscopy; Surface area
Aluminium carbide (Al4C3) is known for its abrasive and creep resistance properties. Generally, it is produced by reaction of aluminium with carbon in electric arc furnace [1]. Al4C3 plays a major role in production of some important structures such as diamond related structures, nanostructure carbons, and growth of diamonds on boron nitride etc. In addition, Al4C3 react with water under high pressure and generates methane [2]. Moreover, Al4C3 particles are used as fine dispersion in aluminium alloy to strengthen the material. In aluminium matrix, Al4C3 particles increase the creep resistance, especially with silicon carbide, which is widely utilizing in automobile and aircraft industries [3]. In order to improve the creep resistance of material, its crystal structure and crystallite size plays an important role. Furthermore, Al4C3 is also used as an abrasive material in cutting tools, where its crystallite size plays a crucial role. After considering the vast importance of Al4C3 in several industries, authors wish to investigate an approach that could be beneficial to modify the physical and structural properties of Al4C3 powder.
Energy is considered as the ability to do work, which interrelates with matter as E=mc2 (Einstein’s famous equation). The energy can effectively interact with any matter at a distance and cause action. In addition, energy also exists with various fields such as electric, magnetic etc. Furthermore, researchers have confirmed that bio magnetic fields are present around the human body, which have been evidenced by electromyography (EMG), Electrocardiography (ECG) and Electroencephalogram (EEG) [4]. Scientists have postulated that it is due to the flow of bioelectricity (generated from heart, brain functions or due to the motion of charged particles such as protons, electrons, and ions) in the human body. As per the basic fundamental law in physics, when an electrical signal passes through any material, a magnetic field is generated in the surrounding space [5]. Due to this, a human has ability to harness the energy from environment/universe and can transmit into any object (living or non-living) around the Globe. The object(s) always receive the energy and responded into useful way that is called biofield energy. This process is termed as biofield treatment. These healing treatments suggest their mechanism upon modulating patient-environmental energy fields [6]. The National Center for Complementary and Alternative Medicine (NCCAM) considered this biofield treatment (therapy) in subcategory of energy therapies [7]. Furthermore, Mr. Trivedi’s unique biofield treatment is known as Trivedi Effect®. Mr.Trivedi’s biofield treatment has substantially altered the physical, structural and atomic characteristic in various metals [8-10] and ceramics [11,12]. Additionally, the influence of biofield treatment was significantly studied in the field of microbiology [13,14], biotechnology [15,16], and agriculture [17-19]. Recently, it was reported that biofield treatment had increased the particle size by six fold and enhanced the crystallite size by two fold in zinc powder [20]. Our group previously reported that biofield treatment has substantial altered the atomic, structural and physical properties in silicon carbide [21] and carbon allotropes [22]. Based on the outstanding results achieved by biofield treatment on metals and ceramics, an attempt was made to evaluate the effect of biofield treatment on physical and structural properties of Al4C3 powder.
The Al4C3 powder was purchased from Sigma Aldrich, India. The sample was equally divided into two parts, considered as control and treated. Treated group was in sealed pack and handed over to Mr. Trivedi for biofield treatment under laboratory condition. Mr. Trivedi provided the biofield treatment through his energy transmission process to the treated group without touching the sample. The control and treated samples were characterized using X-ray Diffraction (XRD), surface area analyzer, and Fourier Transform Infrared Spectroscopy (FT-IR).
XRD analysis of control and treated Al4C3 powder was carried out on Phillips, Holland PW 1710 X-ray diffractometer system, which had a copper anode with nickel filter. The radiation of wavelength used by the XRD system was 1.54056 Å. The data obtained from this XRD were in the form of a chart of 2θ vs. intensity and a detailed table containing peak intensity counts, d value (Å), peak width (θ°), relative intensity (%) etc. Additionally, PowderX software was used to calculate lattice parameter and unit cell volume of Al4C3 powder samples. Weight of the unit cell was calculated as, molecular weight multiplied by the number of atoms present in a unit cell. Density of the unit cell was computed as follows:
The crystallite size (G) was calculated by using formula: G=kλ/ (bCosθ),
Here, λ is the wavelength of radiation used, b is full width half maximum (FWHM) and k is the equipment constant (0.94). Furthermore, the percent change in the lattice parameter was calculated using following equation:
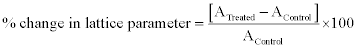
where A Control and A Treated are the lattice parameter of treated and control samples respectively. Similarly, the percent change in all other parameters such as unit cell volume, density, molecular weight, and crystallite size were calculated.
The surface area was measured by the surface area analyser, Smart SORB 90 based on Brunauer–Emmett–Teller (BET), which had a detection range of 0.20–1000 m2/g. Percent changes in surface area were calculated using following equation:
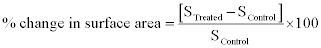
Where, S Control and S Treated are the surface area of control and treated samples respectively.
To see the impact of biofield treatment at bonding level in Al4C3, the FT-IR analysis of control and treated Al4C3 samples was carried out. For FT-IR analysis, Shimadzu, Fourier transform infrared (FT-IR) spectrometer with frequency range of 300-4000 cm-1 was used.
X-ray diffraction (XRD)
XRD analysis results of control and treated Al4C3 samples are illustrated in Table 1 and Figures 1-3. Data showed that the lattice parameter of unit cell was increased by 0.29, 0.24, 0.31, and 0.33% in treated Al4C3 samples T1, T2, T3, and T4, respectively as compared to control [23]. The change in lattice parameter is also known as lattice strain (ε), which is related to stress (σ) by following equation:
| Group | Lattice parameter (Å) | Unit cell volume (×10-22cm3) | Density (g/cc) | Molecular weight (g/mol) | Crystallite size (nm) | |
|---|---|---|---|---|---|---|
| Control | 3.3350 | 2.4012 | 3.013 | 145.234 | 81.56 | |
| Treated, T1 | 3.3446 | 2.4149 | 2.996 | 146.064 | 142.59 | |
| Treated, T2 | 3.3429 | 2.4124 | 2.999 | 145.915 | 190.07 | |
| Treated, T3 | 3.3455 | 2.4162 | 2.994 | 146.143 | 285.08 | |
| Treated, T4 | 3.3460 | 2.4169 | 2.993 | 146.187 | 190.03 |
Table 1: X-ray diffraction analysis of aluminium carbide powder.
σ = Yε
Where, Y is Young’s Modulus
In above equation, negative and positive lattice strain indicates the compressive and tensile stress respectively. Thus, positive strain found in treated Al4C3 sample suggests that biofield treatment might induce tensile stress, which probably stretched the unit cell lattice parameter. Our group previously reported that biofield treatment has altered the lattice parameter in silicon carbide powder [21]. In addition, the lattice strain less than 0.2% is considered as elastic strain, while more than 0.2% is referred as plastic strain [24]. Thus, the positive lattice strain (>0.2%) in treated Al4C3 indicates that biofield treatment probably induced plastic strain. Furthermore, the unit cell volume was increased by 0.57, 0.47, 0.63, and 0.66% in treated Al4C3 samples T1, T2, T3, and T4, respectively as compared to control (Figure 1). Data also showed that density was reduced by 0.57, 0.47, 0.62, and 0.65% in treated Al4C3 samples T1, T2, T3, and T4, respectively as compared to control. Contrarily, the molecular weight of treated Al4C3 was increased from 145.23 g/mol (control) to 146.06, 145.91, 146.14, and 146.18 g/mol in T1, T2, T3, and T4,respectively. It suggest that molecular weight was increased by 0.57, 0.47, 0.63, and 0.66% in treated Al4C3 samples T1, T2, T3, and T4, respectively as compared to control (Figure 2). This could be possible if number of protons and neutrons altered after biofield treatment. Thus, it is hypothesized that a weak reversible nuclear level reaction including neutrons-protons and neutrinos might occurred in treated Al4C3 powders after biofield treatment [25]. It is already reported that biofield treatment has significantly altered the atomic weight and density in silicon dioxide, zirconia [26], and silicon carbide [21]. Besides this, the crystallite size of control and treated Al4C3 powder were computed using Scherrer formula and calculated result are presented in Table 1. Data showed that the crystallite size was increased from 81.56 nm (control) to 142.59, 190.07, 285.08, and 190.03 nm in treated Al4C3 samples T1, T2, T3, and T4, respectively. It suggests that crystallite size of treated Al4C3 powder was significantly increased by 74.83, 133.04, 249.53, and 133.0% in treated Al4C3 samples T1, T2, T3, and T4, respectively as compared to control (Figure 3). Previously, our group reported that biofield treatment has increased the crystallite size in antimony powder [27]. Al4C3 is utilized in aluminium matrix and silicon carbide to increase the creep resistance. Furthermore, Coble proposed that the strain rate in a material is inversely proportional to crystallite size as given below [28]:
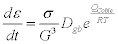
where σ is the applied stress, G is crystallite size, Dgb is diffusion coefficient in grain boundary, QCoble is activation energy for coble creep, R is gas constant, and T is temperature. Coble equation suggests that the strain rate decreases as increase in crystallite size (G) at constant temperature and stress for a given material. Further, the reduction in strain rate increases the creep resistance of a material. In Coble-creep, vacancies and atoms diffused along crystallite boundaries to elongate the crystallite along stress axis to deform the material. Thus, the increase in crystallite size in Al4C3 reduced the crystallite boundaries, which prevents the movement of vacancies along boundaries [29,30]. Shah et al. demonstrated that the creep resistance of metal-carbide was improved after heat treatment due to increase in crystallite size. The increase in crystallite size leads to stabilize the grain boundaries and thus improves creep resistance [31]. In addition, it was demonstrated that grain boundary sliding via slip dominates the creep process in case of finer crystallite size as compared to coarser [32]. Hence, the higher crystallite size found in treated Al4C3 indicates that creep resistance probably enhanced after biofield treatment as compared to control. Therefore, XRD data suggest that biofield treatment has significantly altered the atomic and structural properties in Al4C3.
Surface area analysis
Surface area analysis of Al4C3 powder is presented in Table 2. Data exhibited that surface area of treated Al4C3 powder was increased from 1.60 m2/g (control) to 1.68 m2/g after biofield treatment. This indicates that surface area of treated Al4C3 powder was slightly increased by 5.0% as compared to control. Our group previously reported that biofield treatment has significantly reduced the particle size and increased the surface area in zirconium oxide [26]. Thus, it is assumed that the increase of surface area in treated Al4C3, possibly due to particle size reduction after biofield treatment. The existence of internal strains in treated Al4C3 was evidenced by XRD data (Figure 1), which might induce fractures in particles and reduced size. Hence, it is concludes that biofield treatment has altered the physical characteristics of Al4C3 powder as compared to control.
| Surface Area (m2/g) | Percent change | |
|---|---|---|
| Control | Treated | |
| 1.60 | 1.68 | 5.0 |
Table 2: Surface area analysis of aluminium carbide powder.
FT-IR analysis
FT-IR spectra of control and treated Al4C3 samples are illustrated in Figure 4. In control Al4C3 samples absorption peaks were observed at 499, 609, 711, and 785 cm-1, which could be due to Al-C bonding vibrations. The control data is well supported by literature data [33]. The treated Al4C3 also showed similar absorption peaks at 499, 609, 709, and 785 cm-1, which could be assigned Al-C bonding vibrations. Furthermore, peaks observed at 1490 and 1440 cm-1 in control and treated Al4C3 respectively, could be due to moisture absorption. In addition, the peaks observed at 2358 and 2395 cm-1 in control and treated Al4C3 respectively, could be due to CO2 absorption by samples. Thus, FT-IR data suggest that no significant change was observed in absorption peaks of treated Al4C3as compared to control.
Biofield treatment showed an increased lattice parameter and unit cell volume of treated Al4C3samples upto 0.33 and 0.66% respectively, as compared to control. It may be due to tensile stress, which probably generated in treated Al4C3 samples after biofield treatment. In addition, the molecular weight was increased upto 0.66% in treated Al4C3 samples as compared to control. It is hypothesized that biofield treatment may induce nuclear level reaction, which resulted into increase of molecular weight in treated Al4C3 sample. Besides, the crystallite size of treated Al4C3samples was significantly increased upto 285.08 nm from 81.56 nm (in control). The increase in crystallite size could improve the creep resistance and abrasive properties of treated Al4C3samples. Furthermore, the surface area was increased by 5% in treated Al4C3 samples as compared to control. It could be due to alteration of shape/ size of Al4C3 particles after biofield treatment. However, no significant change was observed in absorption peaks in FT-IR spectra of treated Al4C3 as compared to control. Therefore, based on above outcomes of XRD and surface area analysis, it is assumed that treated Al4C3with high creep resistance could be more useful in automobile and aircraft manufacturing industries.
Authors gratefully acknowledged to Dr. Cheng Dong of NLSC, Institute of Physics, and Chinese academy of Sciences for providing the facilities to use PowderX software for analyzing XRD data. Authors also would like to thank Trivedi science, Trivedi master wellness and Trivedi testimonials for their support during the work.