Poultry, Fisheries & Wildlife Sciences
Open Access
ISSN: 2375-446X
ISSN: 2375-446X
Research Article - (2017) Volume 5, Issue 1
The present study is a characterization of the chemical composition of crab shells from brown crab, spider crab, velvet crab and green crab. The chitin content of crab shells varied between 9.7 and 16.4% and the protein content was in the range of 13.2 to 20.7%. Ash was the major constituent and accounted for more than 70%. The total carotenoid content ranged between 0.6 and 9.3 μg/g depending on the crab species. Concerning macro elements their content followed the descending order in all species: Ca>P>S>Sr>Cl>K. In the case of trace elements content the descending order in brown crab was: Br>Fe>Rb>Cu>Zn; in spider crab was: Br>Fe>Rb>Zn>Cu and in velvet and green crab was: Br>Fe>Zn>Rb>Cu. The level of contaminants was relatively low and the descending order of their content was the following: As>Pb>Cd>Hg. Crab shells are a potential source of chitin and the levels of macro and trace elements together with the low contaminants concentration make them a raw material for chitin production or utilization as feed ingredients or fertilizers.
<Keywords: Crab exoskeleton; Chemical composition; Chitin; Protein; Carotenoids; Mineral fraction
The crustacean exoskeleton presents several functions including the stabilization of the whole body of the animal, resistance to mechanical loads, and protection to the environment and against predators [1]. The structure of crustacean exoskeleton consists of an organic matrix constituted by α-chitin, proteins and carotenoid pigments and an inorganic fraction where calcium carbonate is the main constituent [2]. The relative percentages of these constituents depend on the species and within a certain species they present seasonal changes and also vary among different parts of the skeleton [3]. The crustacean skeleton is a byproduct from processing of crustaceans and represents a valuable raw material for different applications. Crustacean shells have been utilized in the preparation of fish feeds [4,5] and broilers [6]. The presence of chitin in crustacean shells may be a relevant factor as it is recognized its role in activation the innate immune system of fish [4]. This muco polysaccharide polymer can also modulate the fish gut microbiota [7]. Moreover, this by-product is also a source of carotenoid pigments [8] as well as the main raw material for the extraction of chitin [9]. Its utilization to uptake and removal of metal ions in solution was reported [10] but it can be simply used for composting [11]. About 20% of all crustaceans caught or farmed worldwide are crabs. Brown crab (Cancer pagurus), European spider crab (Maja squinado), green crab (Carcinus maenas), and velvet crab (Necora puber) are the most popular crab species consumed in Europe. Brown crab landings attained around 51,247 tonnes in 2014 where those in the United Kingdom represented more 60% of total landings [12]. The European spider crab landings were around 6,538 tonnes in 2014, mainly in France [12]. Green crab landings in 2014 were approximately 1453 tonnes, mostly in France and United Kingdom [12]. The landings of velvet crab were around 2448 tonnes in 2014, mainly in United Kingdom [12].
The available information on crustacean waste is very scarce. In one report published by Sea Fish Industry Authority is estimated a quantity of 3,500 to 7,000 tonnes of crab wastes assuming that 25% to 50% of whole crabs are processed in UK [13].
The objective of this work was to evaluate the chemical composition of the shell of brown, spider, green, and velvet crabs. The chemical characterization of shells involved the determination chitin, protein and ash content, total carotenoids and mineral fraction (macro and trace elements and contaminants).
Brown crab males and females (Cancer pagurus) caught during spring (n=24), summer (n=20), autumn (n=20) and winter (n=20) in the Scottish coast and during summer in French waters (n=18) were purchased alive from a local importer. Males and females from green crab (Carcinus maenas, n=48) and velvet crab (Necora puber, n=46) caught in the Scottish coast during summer and spider crab (Maja squinado, n=20) during autumn were purchased alive from a local importer. The muscle was separated and shells were hand washed with hot tap water to remove flesh residues, lipids and other materials. Washed and dried shells were crushed to small pieces or powdered and stored at -20°C until further analysis.
Chitin extraction
Chitin was extracted by acid treatment (demineralization) followed by alkaline protein extraction (deproteinization) [9]. The quantity of the chitin expressed as percentage of the shell was calculated after this process.
Protein content
The protein content of brown crab shell was calculated by the following formula:
P (%)=100–(Chitin (%)+Ash (%)).
Carotenoid content
Total carotenoids were measured by UV/Vis spectrophometry at a wavelength of 468 nm. One gram of ground crab shell was extracted four times with 5 mL of acetone for 30 min. and all extracts were pooled and 2.5 mL of water added. The carotenoids were extracted twice with 10 mL of hexane. Both hexane extracts were pooled and 5 mL of a 5% NaCl solution were added and dried with anhydrous Na2SO4. The total of carotenoids content (using astaxanthin as a standard) in the extracts was calculated using the formula:
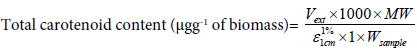
where A is the absorbance at 468 nm, Vext is the volume of the extract, MW is the molecular weight of astaxanthin 596.84 (g/mol), 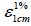 is the coefficient of extinction of astaxanthin 124000 mol-1L.cm-1 and Wsample is the weight of the sample (g).
is the coefficient of extinction of astaxanthin 124000 mol-1L.cm-1 and Wsample is the weight of the sample (g).
Ash content
Ash content was determined according to the AOAC methodology [14].
Elemental analysis and contaminants (Hg, Cd and Pb)
Energy dispersive X-ray fluorescence (EDXRF) was employed to quantify Cl, S, K, Ca, Fe, Cu, Zn, As, Br and Sr [15]. Sodium, cadmium and lead were quantified by Flame atomic absorption spectrometry (FAAS) in a Spectra AA 20 spectrometer (Varian Australia, Mulgrave, Victoria, Australia) [15]. Mercury was analyzed by atomic absorption spectrophotometry according to test method 7473 [16] using an Hg analyzer Leco, AMA 254. In all analyses, a minimum of three replicates was performed per sample.
Chitin content
No significant differences between the chitin content of males and females were recorded in the shells of all species except females harvested in Scotland during the spring which have higher chitin content than males (Figure 1A). Furthermore, the chitin content of spider crab was significantly higher than that of other crab species. The chitin content of crab shell presents a wide variation within these species but the results obtained in the current work were of the same order of magnitude of those reported by other authors. Thus, chitin content in the range of 12.6% and 15% (dw) was reported for green crab shell [17,18]. Higher chitin content (27.4%) was referred for the shell of Carcinus mediterraneus [19]. Different sections of the snow crab were analysed and obtained levels of chitin between 18.70% and 32.25% [8]. On the other hand, lower chitin content (6.83%) was determined in a freshwater crab shell [20].
Figure 1: Chitin content (A), Protein content (B), Total Carotenoids (C) and Ash content (D) of Velvet Crab (VC), Green Crab (GC), Spider Crab (SC) and Brown Crab (BC) male and female shells from scottsih (Scot) and French (fr) waters harvested in different seasons (1-autumn, 2-winter, 3-spring, 4-summer). Means without a common letter differ significantly (p<0.05).
Protein content
The protein content of the different crab species shells was in the range of 13.2% and 20.7% (Figure 1B). The level of this constituent in the male shell of all species was similar to that of female shells with exception of winter brown crab harvested in Scotland. Lower protein content between 4.31 and 7.06% was reported in the green crab shell depending on the harvesting site [18]. Relatively low protein content (8.0-12.7%) also obtained for snow crab shell which depended on the crab shell part analysed [21]. On the other hand, higher protein content (34.2%) was reported in snow crab shell [22]. These differences in the protein content may result from the method of processing crabs [18]. The protein content reported by for the shell of two crab species was in the range of values obtained in this study [23].
Total carotenoids content
The lowest total carotenoids values (p<0.05) were measured in brown crab and spider crab (Figure 1C). Moreover, no significant differences between males and females of brown crab and spider crab were observed. However, green crab males had higher content of total carotenoids than females. Concerning samples from Scotland and France harvested in summer, no significant differences in the level of these constituents were recorded. A total carotenoids content range between 4 μg/g [24] and 14 μg/g [18] was reported. The carotenoids content depends on the crab species, harvesting season and site as well as on the extraction conditions, particularly the solvent used in the extraction, and the conditions and storage time may have also influence the yield achieved.
Ash content
Ash was the major constituent of crab shells and accounted for about 70% (Figure 1D). The highest ash content was recorded in the brown crab (74.97%) and the lowest in spider crab (62.90%) whereas velvet crab and green crab carapaces had intermediate values. There were no significant differences between the ash content of males and females with exception of autumn brown crab harvested in Scotland. Regarding shells from brown crabs harvested in Scotland and France in the summer no significant differences were observed within these samples. These values are considerable higher than those obtained for crab shells by other authors. An ash content of 30.6% (dw) was reported for the snow crab offals [21] and the percentages of 58.6 and 40.6 were indicated for blue crab shell and snow crab shell, respectively [25]. For the crab species Callinectes pallidus and Cardisoma armatus it was reported an ash content of 46.01% and 56.36%, respectively [23] and a percentage of 59.8% (dw) for Carcinus mediterraneus [19].
Macro elements content
Calcium and phosphorous were the most important elements followed by sulphur (Table 1). Calcium is basically incorporated in calcium carbonates in the form of calcite or amorphous calcium carbonate [1]. The brown crab shells were richer in calcium than the shells of the other crab species and the lowest content of this element was in the spider crab. No significant differences between the calcium content of male and female shells were observed. In an early study [26] was reported a calcium concentration of 294.3 mg/g and 247.1 mg/g in the exoskeleton of brown and green crab, respectively and Boβelmann et al. [3] indicated a calcium content of 220 mg/g for brown crab. A calcium level of 16.55% was indicated for the green crab shell [27].
| Species | K (mg/g) | Ca (mg/g) | Sr (mg/g) | P (mg/g) | S (mg/g) | Cl (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | |
| BCscot1 | 1.9 (0.6) |
2.2 (0.0) |
439.1 (27.5) |
474.1 (19.7) |
5.1 (0.6) |
4.8 (0.2) |
290.2 (12.5) |
309.6 (3.0) |
20.3 (2.4) |
23.2 (0.2) |
4.6 (3.5) |
9.8 (0.7) |
| BCscot2 | 2.1 (0.1) |
2.0 (0.2) |
519.5 (44.2) |
504.6 (38.1) |
5.2 (1.12) |
5.7 (1.1) |
322.1 (19.1) |
321.8 (19.5) |
23.7 (0.6) | 23.2 (2.7) |
3.2 (0.0) |
5.4 (1.8) |
| BCscot3 | 2.2 (0.1) |
1.6 (0.4) |
512.1 (22.8) |
432.2 (62.2) |
5.2 (0.7) |
5.1 (0.4) |
326.1 (7.3) |
286.4 (31.9) |
22.9 (1.2) |
15.5 (5.1) |
2.8 (0.0) |
3.1 (1.7) |
| BCscot4 | 2.0 (0.0) |
1.9 (0.5) |
513.9 (55.2) |
566.7d (6.3) |
4.9 (0.7) |
4.7 (0.0) |
317.6 (19.8) |
344.3 (0.1) |
22.7 (0.4) |
25.5 (2.6) |
5.7 (1.7) |
2.7 (0.6) |
| BCfr4 | 1.8 (0.3) |
1.3 (0.2) |
486.8 (21.8) |
432.0 (35.6) |
4.7 (0.1) |
5.4 (1.5) |
310.0 (10.8) |
286.0 (28.6) |
21.3 (1.2) |
19.5 (0.5) |
2.4 (0.0) |
1.9 (0.1) |
| SC | 2.0 (0.3) |
2.2 (0.3) |
308.2 (34.5) |
332.4 (24.8) |
5.2 (0.7) |
5.2 (1.1) |
229.1 (19.6) |
242.3 (4.84) |
13.4 (3.0) |
16.7 (5.0) |
7.8 (5.7) |
7.6 (6.7) |
| VC | 2.7 (0.9) |
--- | 326.6 (70.8) |
--- | 4.8 (0.2) |
--- | 241.6 (33.4) |
--- | 14.8 (4.8) |
--- | 11.0 (3.3) |
--- |
| GC | 2.8 (0.2) |
2.7 (0.7) |
397.5 (1.3) |
399.6 (91.0) |
4.7 (0.4) |
5.5 (0.7) |
272.5 (8.0) |
267.1 (36.8) |
19.0 (2.1) |
17.8 (5.4) |
4.1 (0.7) |
7.9 (6.8) |
M: male; F: female
In brackets is shown the standard deviation
Table 1: Levels of macroelements in the male and female shells of spider crab (SC), velvet crab (VC), green crab (GC), and brown crab from Scottish (BCscot) and French (BCfr) waters harvested in different seasons (1-autumn, 2-winter, 3-spring, 4-summer).
Phosphorous represented 23-34 % of the shell weight of all crab shells analysed (Table 1). Such as the calcium distribution in the shell of the various crab species, the highest phosphorous content was measured in brown crab and the lowest in the spider crab. Likewise calcium content the level of phosphorous in male and female shells was not significantly different. The level of this element in the crab shell presents some variation in the same species, in the different parts of the shell and among species [3,21,22,28,29].
The potassium content range of the crab samples was relatively narrow and varied between 1.3 and 2.8 mg/g where the maximum value was recorded in the male of green crab and the minimum in the female of brown crab harvested in France. Moreover, no significant differences in the levels of this element were recorded. Higher potassium content was referred for freshwater crab (9 mg/g in females and 11 mg/g in males) [29] and in Callinectes pallidus (6.05 mg/g) [23]. However, the latter authors obtained only a potassium level of 1.02 mg/g in the shell of Cardisoma armatum.
The levels of strontium in all species varied between 4.7 and 5.5 mg/g and no significant differences were observed among them. The mean percentage of this element in the exoskeleton of the crab species analysed was similar to that reported for brown crab and green crab [26] as well as to the percentage obtained in snow crab offals [21].
In general brown crab shell presented sulphur content higher than 20 mg/g and the lowest values were measured in spider and velvet crabs. The sulphur detected in the exoskeleton of the crab species analysed may be in the form of sulphates [30] but also in the sulphur-containing amino acids present in the cuticular proteins [31].
The lowest (1.9 mg/g) and the highest (11.0 mg/g) chlorine content were measured in the carapace of brown crab females and in the carapace of velvet crab males. The level of this element becomes predominant in the tips of tarsal claws of crabs when the level of calcium decreases [32]. A significant mass of chlorine was detected in the chelipeds and leg tips of grapsid crab but only traces in the carapace [33].
Trace elements
A wide range of variation (20.7-123.1 μg/g) of rubidium content was recorded in the different crab species (Table 2). The highest content was measured in the velvet crab (123.1 μg/g). A rubidium level of 1.06- 19.6 μg/g was detected in the exoskeleton of giant river prawn [34].
| Species | Rb(mg/g) | Fe(mg/g) | Cu(mg/g) | Zn(mg/g) | Br(mg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | |
| BCscot1 | 29.2 (7.9) | 70.1 (3.8) | 75.8 (14.2) | 144.3 (0.1) | 22.4 (3.1) | 37.2 (5.7) | 4.0 (0.6) | 23.3 (6.3) | 435.0 (140.1) | 606.8 (30.2) |
| BCscot2 | 56.8 (36.0) | 108.6 (18.7) | 108.6 (10.0) | 85.8 (7.0) | 22.9 (2.7) | 35.5 (1.4) | 12.0 (4.7) | 24.1 (3.2) | 630.6 (13.3) | 757.1 (91.3) |
| BCscot3 | 99.7 (19.8) | 89.2 (16.4) | 154.9 (5.4) | 121.5 (22.4) | 34.3 (10.9) | 24.1 (2.7) | 20.0 (3.2) | 6.8 (1.2) | 712.5 (61.3) | 837.3 (47.3) |
| BCscot4 | 85.6 (6.4) | 81.4 (5.2) | 274.3 (17.3) | 115.8(3.8) | 32.1 (3.2) | 32.2 (5.2) | 22.2 (3.7) | 12.7 (2.9) | 743.8 (19.4) | 785.6 (50.3) |
| BCfr4 | 64.3 (8.1) | 45.6 (24.6) | 107.2 (2.2) |
72.8 (20.5) |
20.9 (4.2) |
19.8 (1.6) |
19.6 (3.0) |
10.7 (2.8) |
636.3 (42.6) |
498.8 (113.7) |
| SC | 33.1 (11.0) | 24.9 (11.4) | 668.0 (113.8) |
401.2 (2.9) |
22.5 (2.3) |
20.5 (0.7) |
29.4 (1.0) |
23.9 (2.8) |
1161.7 (43.0) |
956.0 (70.0) |
| VC | 123.1 (31.9) |
--- | 693.5 (43.9) |
--- | 22.9 (0.9) |
--- | 178.2 (21.2) |
--- | 3710.5 (1209.8) |
--- |
| GC | 28.6 (0.2) |
20.7 (4.0) |
175.7 (77.0) |
542.8 (84.1) |
18.0 (0.3) |
17.3 (3.4) |
24.4 (7.0) |
29.2 (7.1) |
371.6 (155.1) |
453.2 (59.2) |
M: male; F: female
In brackets is shown the standard deviation
In each column different letters indicate significant differences per element
Table 2: Levels of trace elements in the male and female shells of spider crab (SC), velvet crab (VC), green crab (GC) and brown crab from Scottish (BCscot) and French (BCfr) waters harvested in different seasons (1-autumn, 2-winter, 3-spring, 4-summer).
Iron content presented a wide range of variation both within species and between species. In the brown crab the level of this element varied between 72.8-154.9 μg/g. On the other hand, the iron content in the shell of spider, velvet and green crab females was almost four fold higher than the highest content obtained in brown crab. The level of iron in green crab obtained in the current study, particularly in the female shell, was higher than that reported by Bjerregaard and Depledge [35]. Furthermore, the range of iron content of brown crab shell was similar to the level reported in snow crab offals [21]. The high variation of iron content was also reported in other crustacean species [23,29].
The levels of copper content in the exoskeleton of the different crab species varied between 17.3 and 37.2 μg/g where the lowest value were obtained in green crab. Slightly lower copper content (11.0 and 5.7 μg/g for small and large crabs, respectively) was determined in green crab carapace [35]. A relatively low copper content was also reported for snow crab offals [21] and a benthic crab [36], respectively. However, higher levels of this element were reported in the exoskeleton of other crab species [23,37].
There are considerable differences in the zinc content in the same species and interspecies (Table 2). The intra species variation is quite noticeable in brown crab and, on the other hand, the high content of this element in the velvet crab exoskeleton (178.2 μg/g) stands out from all other crabs evidencing the interspecific changes. The level of zinc in green crab shell was about the double of that obtained by other authors [35]. Low levels of this element were also reported in different crab species [23,29]. Conversely, very high zinc content in other crab species [36,38].
The exoskeleton of the crab species analysed presented relatively high bromine levels, particularly in velvet crab (3.7 mg/g) which was also the richest in chlorine. High concentrations of bromine are found in invertebrates incorporated in bromine-rich material present in the tips of cuticular structures [33,39]. The advantage of this bromine-rich cuticle over calcified cuticle is its resistance to fracture [39]. According to these authors it seems that bromine is bound to phenyl rings.
Contaminants
Low levels of arsenic were detected and no significant differences were obtained among the different crab shell species being the highest concentration measured (13.1 μg/g) in female shell of green crab (Table 3). Levels (ww) of about 2.2 μg/g and 1.2 μg/g of this element were obtained in the exoskeleton of green crab females and males, respectively [40]. Conversely, considerable higher levels of this contaminant were reported in snow crab offals (27 μg/g) [21].
| As (mg/g) | Cd (mg/g) | Pb(mg/g) | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| BCscot1 | 8.7 (0.7) |
8.2 (2.4) |
0.010 (0.016) |
< LQ | 0.141 (0.071) |
0.153 (0.013) |
| BCscot2 | 7.4 (3.5) |
10.9 (1.1) |
0.045 (0.001) |
0.009 (0.001) |
< LQ | 0.083 (0.030) |
| BCscot3 | 7.5 (3.8) |
10.1 (0.7) |
0.069 | 0.016 | 0.188 (0.042) |
< LQ |
| BCscot4 | 11.6 | 13.0 (1.4) |
0.020 (0.011) |
< LQ | 0.219 (0.144) |
< LQ |
| BCfr4 | 6.0 (0.8) |
5.7 (0.9) |
0.021 (0.001) |
0.016 (0.001) |
0.236 (0.053) |
0.201 (0.001) |
| SC | 7.3 (0.4) |
8.7 (4.6) |
0.012 (0.001) |
0.010 (0.007) |
< LQ | 0.340 (0.04) |
| VC | 2.3 (0.6) |
--- | 0.106 | --- | 0.396 | --- |
| GC | 4.0 (1.8) |
13.1 (4.0) |
0.036 (0.006) |
< LQ | 0.332 | 0.270 |
LQ – Limit of quantitation; Cd, LQ = 0.005 ?g/g; Pb, LQ = 0.08 ?g/g. In brackets is shown the standard deviation.
Table 3: Content of As, Cd and Pb in the male and female shells of spider crab (SC), velvet crab (VC), green crab (GC), and brown crab from Scottish (BCscot) and French (BCfr) waters harvested in different seasons (1-autumn, 2-winter, 3-spring, 4-summer).
Concerning cadmium (Table 3), lower levels were generally found in the female shells and the highest concentration was registered in the carapace male of velvet crab. The cadmium levels in the green crab exoskeleton [35] were below the detection limit but considerably higher cadmium levels were obtained (0.4-18.5 μg/g, ww) in the carapace of the freshwater crab Potamonautus perlatus [41] as well as in the exoskeleton of blue swimming crab (12.34-20.30 μg/g, dw) [37].
In the case of lead, the highest level of this contaminant was detected in the shell of velvet crab males (Table 3) which also presented the highest level of cadmium. In brown crab exoskeleton the level of lead was below the limit of quantification in some samples and attained a maximum of 0.236 ± 0.053 μg/g in the exoskeleton sample of crab male harvested in French waters. A big difference between the lead content of male and female shells was also detected in spider crab. The content of lead in the carapace of freshwater crab presented a wide variation between 0.7 and 327.6 μg/g [41]. According to these authors lead is incorporated mainly into the exoskeleton of crustaceans and periodic molting provides a possible route for eliminating contaminants. Similar values of lead content in the range of 0.05-1.90 μg/g were reported in the exoskeleton of blue swimming crab [37,42].
The levels of mercury in the crab shells were below of detection limit (0.005 μg/g). However, other authors have detected this contaminant in the crustacean exoskeleton. Thus, low levels of this contaminant were obtained in the exoskeleton of green crab, approximately 0.008 μg/g in males and 0.003 μg/g in females [43]. Considerably higher concentrations of mercury were determined in the exoskeleton of the blue swimming crab [42,44]. The former authors [42] obtained values in the range of 0.32-1.30 μg/g with a mean value of 0.69 μg/g and the latter authors [44] referred a variation between 0.40 and 1.41 μg/g and mean value of 0.71 μg/g. The differences in the mercury content recorded in the exoskeleton of the various species are certainly related to the mercury contamination of the harvesting/sampling area.
The chitin content of the crab shells analysed indicates that they can be used as a source for the extraction of this valuable biopolymer or for the metallic ions removal from solutions. The levels of carotenoids in these shells were relatively low and they cannot be considered a good source of these pigments. The high ash content of the crab shells make them useful as fertilizers or feed ingredients, particularly due to the levels of calcium and potassium. On the other hand, they can be considered safe ingredients due to their low levels of contaminants.
Acknowledgements
The authors acknowledge the project ACRUNET “Transnational Approach to Competitiveness and Innovation in the Brown Crab Industry“(2011-1/148).