Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2021)Volume 10, Issue 9
Phytochemical investigation of dichloromethane extract of the fruit of Cucumis ficifolius led to the isolation of 2', 3'- dihydroxypropyl pentadecanoate (1), pentadecanoic acid (2) and tetradecanoic acid (3). The structure of the compounds was established based on 1D and 2D NMR spectroscopic studies and comparison with literature data. This is the first report to isolate and characterize secondary metabolite from Cucumis ficifolius. The crude extract and the compounds were evaluated for their antibacterial activity against five bacterial strains (S. aures, E. coli, P. aueroginosa, S. tphyimurium and S. flexineri). The crude extract showed considerable activity, whereas the isolated compounds showed little or none inhibitory activities against the test bacterial strains, which could be due to the synergetic effects of the various kinds of compounds in the crude extracts or a compound responsible for the activity of the crude extract could be minor and has not been isolated.
Medicinal plant, Cucumis ficifolius, 2', 3'-Dihydroxypropyl pentadecanoate, Pentadecanoic acid, Tetradecanoic acid, Antibacterial activity
The genus Cucumis, Linnaeu (family Cucurbitaceace) comprises of 55 species widely distributed in Africa, India, Southeast Asia and Australia [1, 2]. It is one of the most important plant families supplying humans with edible products and useful fibers. They are also well-recognized for their source of ubiquitous class of bioactive constituents of the human diet, manifesting both nutritional and health benefits [3]. Cucumis ficifolius is one of the wild plants of the genus commonly used in Ethiopia for the treatment of different alignments [4, 5]. The roots part of C. ficifolius is well known for its curative property of livestock diseases [6-13]. The water juice of the root was used as rabies virus vaccination [6, 7, 10, and 14-20]. It has also been reported that the fruit juice is widely used for anti-nociceptive, anti-inflammatory effects [5] and antibacterial activities [4].
Despite the wider use of this plant by the communities, to the best of our knowledge, there is no phytochemical analysis report pertaining to this plant. Therefore, this study was initiated to isolate, characterize and evaluate biologically active compounds from the fruit of C. ficifolius; and here we are reporting the isolation of three compounds (Fig.1) from the fruit of C. ficifolius.
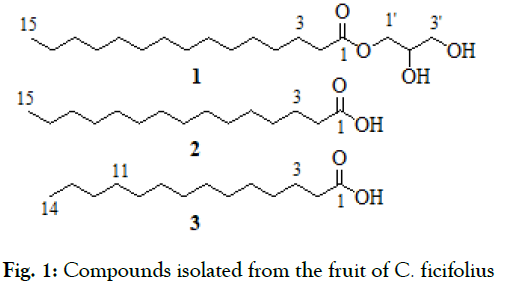
Fig. 1: Compounds isolated from the fruit of C. ficifolius.
Instruments and chemicals
Solvents and reagents used for extraction and purification of the compounds are of analytical and HPLC grade. Analytical TLC pre-coated sheets ALUGRAM®Xtra SIL G/UV254 (layer: 0.20mm silica gel 60 with fluorescent indicator UVF254/365) was used for purity analysis. For column chromatography, silica gel 100-200 mesh was used. Chromatograms were visualized on TLC by spraying with 10% H2SO4 acid and heating on hot plate. NMR spectra were recorded on an Avance 500 MHz spectrometer (Bruker, Billerica, MA, USA, at 500MHz (1H) and 125MHz (13C)). Chemical shifts were expressed in parts per million (ppm) downfield of Trimethylsilane (TMS) as internal reference for 1H resonances, and referenced to the central peak of the appropriate deuterated solvent's resonances (residual CDCl3 at δH 7.26 for protons and δC 77.0 for carbons).
Bacterial strains
One gram-positive bacterial strains (Staphylococcus aureus (ATCC 25923)) and four gram-negative bacterial strains (Escherichia coli (ATCC 25922), Pseudomonas aueroginosa (ATCC-27853), Salmonella tphyimurium (ATCC 13311) and Shigella flexineri (ATCC 29903)) were obtained from Wollega university of biology department and were used to evaluate the antibacterial activities of the isolated compounds.
Antibacterial activity assay
Paper disc diffusion method was employed during this study to determine the antibacterial activity of the isolated compounds as described by [21-23] with slight modification against one grampositive bacterial strains (Staphylococcus aureus (ATCC 25923)) and four gram-negative bacterial strains (Escherichia coli (ATCC 25922), Pseudomonas aueroginosa (ATCC-27853), Salmonella tphyimurium (ATCC 13311) and Shigella flexineri (ATCC 29903)) which were grown on a nutrient agar medium (3g/L beef extract, 10g/L peptone and 20g/L agar) and the pH was adjusted to 7.2. 20ml of seeded medium was poured into sterile Petri dishes and allowed to solidify and dry. Each piece of sterile paper discs (diameter 6-mm) impregnated with 0.5mg/ml isolated compounds, gentamycin (standard solution) each were applied to the Petri dishes of microbial culture and kept in a refrigerator at 40C for 2hr to let the applied substances to diffuse to the agar and then incubated at 350C for 24hr. All tests were performed in triplicate and zone of inhibition were measured from the edge of each disc after the incubation period.
Plant materials
The pre-identified C. ficifolius fruits from secondary data and local practitioners were collected in March, 2019 from Wollega University campus, Nekemte, Oromia, Ethiopia. The plants were authenticated by Dr. Fikedu Gurmessa and a voucher specimen (TTA001Cf) was deposited in Biology Department of Wollega University. The collected plant part was washed thoroughly with tap water and cut into small pieces and dried in organic laboratory till constant weight is obtained at room temperature (25 0C) and finally grinded to a fine powder using laboratory mill.
Extraction and isolation of compounds 1-3
The fine powder of C. ficifolius fruit (480 g) was extracted by dichloromethane(3x1L) at room temperature for three times each 24 hr each with occasional shaking and finally filtered the crude from marc using cotton cloth and then with Whatman filter paper to remove unnecessary substances to yield 10 g of brown solid.
About 8 g of the crude extract was adsorbed with silica gel and subjected to column chromatography (3.4 cm diameter, 40cm length and 200g capacity) packed with 175g of silica gel (100-200 mesh size). The column was eluted with a mixture of hexane and dichloromethane (70:30, v: v) and carried out with increasing polarity of dichloromethane to100%. Then, followed with the mixture of dichloromethane and methanol (90:10, 80:20… 0:100, v: v) solvent system. About seventy three fractions each of ca. 50 mL were collected. Fractions of similar TLC profiles were combined and six sub-fractions (DCF1-6) of fractions were obtained. Sub-fraction DCF6 showed two spots on TLC after sprayed with 10% H2SO4 solution and heated on hot plate. The precipitate was observed after the solution left open for a night. Then, the precipitate was washed repeatedly with n-hexane and finally afforded a white powder, 1 (20 mg). While, sub-fraction DCF4 contained a mixture of compounds which was further purified by small column chromatography on silica gel (eluent: increasing gradient of dichloromethane in hexane) and resulted compound 2 (40 mg) and 3 (10 mg).
Spectroscopic data of compounds 1-3
2', 3'-Dihydroxypropyl pentadecanoate (1) obtained as white precipitate. The 1H and 13C NMR data, see Table 1
Pentadecanoic acid (2) obtained as white solid. The 1H and 13C NMR data, see Table 1
Tetradecanoic acid (3) obtained as white solid. The 1H and 13C NMR data, see Table 1
| No | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| (δmH, j Hz) | δC, C- type | δH(m, J Hz) | δC, C-type | δH(m, J Hz) | δC, C- type | |
| 1 | - | 174.4, C | - | 180.5, C | - | 179.0, C |
| 2 | 2.37 (t, 7.6) | 34.2, CH2 | 2.34(t, 7.5) | 34.5, CH2 | 2.35(t, 7.5) | 2.35(t, 7.5) |
| 3 | 1.65 (q, 7.3) | 24.9, CH2 | 1.62(m) | 25.1, CH2 | 1.63(q, 7.5) | 24.7, CH2 |
| 4 | 1.30-1. 35(m) | 29.1, CH2 | 1.23-1. 29(m) | 30.1, CH2 | 1.23-1 33(m) | 29.7, CH2 |
| 5 | 1.30-1. 35(m) | 29.7, CH2 | 1.23-1. 29(m) | 30.1, CH2 | 1.23-1 33(m) | 29.7, CH2 |
| 6 | 1.30-1. 35(m) | 29.7, CH2 | 1.23-1. 29(m) | 30.1, CH2 | 1.23-1 33(m) | 29.7, CH2 |
| 7 | 1.30-1. 35(m) | 29.7, CH2 | 1.23-1. 29(m) | 30.0, CH2 | 1.23-1 33(m) | 29.6, CH3 |
| 8 | 1.30-1. 35(m) | 29.7, CH2 | 1.23-1. 29(m) | 29.9, CH2 | 1.23-1 33(m) | 29.5, CH2 |
| 9 | 1.30-1. 35(m) | 29.6, CH2 | 1.23-1. 29(m) | 29.6, CH2 | 1.23-1 33(m) | 29.4, CH2 |
| 10 | 1.30-1. 35(m) | 29.5, CH2 | 1.23-1. 29(m) | 29.8, CH2 | 1.23-1 33(m) | 29.3, CH2 |
| 11 | 1.30-1. 35(m) | 29.4, CH2 | 1.23-1. 29(m) | 29.6, CH2 | 1.23-1 33(m) | 29.1, CH2 |
| 12 | 1.30-1. 35(m) | 29.3, CH2 | 1.23-1. 29(m) | 29.5, CH2 | 1.23-1 33(m) | 31.9, CH2 |
| 13 | 1.30-1. 35(m) | 31.9, CH2 | 1.23-1. 29(m) | 32.3, CH2 | 1.23-1 33(m) | 22.7, CH2 |
| 14 | 1.30-1. 35(m) | 22.7, CH2 | 1.23-1. 29(m) | 23.1, CH2 | 0.88(t, 5.0) | 14.1, CH3 |
| 15 | 0.90 (t, 6.8) | 14.1, CH3 | 0.87(t, 6.8) | 14.5, CH3 | ||
| 16 | 4.23 (dd, 11.7, 4.6) | 65.2, CH2 | ||||
| 17 | 3.97(m) | 70.3, CH | ||||
| 18 | 3.97(m) | 63.3, CH2 | ||||
Table 1: 1H (500MHz) and 13C (125MHz) NMR data of compounds 1, 2 and 3 (in CDCl3)
Chromatographic separation of dichloromethane extract of C. ficifolius fruit was carried out and lead to the isolation of three compounds: 2',3'-dihydroxypropyl pentadecanoate (1), pentadecanoic acid (2) and tetradecanoic acid (3). The structural elucidations of these compounds were done based on the spectroscopic data obtained from 1D and 2D NMR and by comparing with the literature values of similar compounds.
Compound 1 was isolated as white precipitate. The 1H-NMR spectrum in CDCl3 (Table 1) showed characteristic peaks for long chain fatty acid derivatives, these include the triplet peak at δH 2.37 (t, J = 5.0 Hz) for two protons attributed to the protons adjacent to carbonyl group and another pentet (quintet) peaks at δH 1.65 (q, J = 7.3 Hz) of two protons that indicated the presences of aliphatic methylene protons of C-3. Moreover the over lapped multiple peaks in 1H-NMR spectrum in the interval of δH 1.30-1.35 is also a typical properties of aliphatic protons which are integrated for twenty two protons. The up filed signal in 1H-NMR spectrum at δH 0.90 (t, J = 6.8 Hz) designated the presences of terminal methyl protons (C-15) (Khan et al., 2013). The doublet of doublet peaks at δH 4.23 (dd, J = 11.7, 4.6 Hz) and at δH 3.62 (dd, J = 11.5, 5.8 Hz) are due to nonequivalent geminal protons on C-1' (CH2) and C-3' (CH2OH) respectively, which are attributable to diastereotopic protons on C-1' (CH2) at δC 65.2 and C-3' (CH2OH) at δC 63.3 that is supported by HMQC experiment. The proton decoupled 13C NMR (Table 1) spectrum showed the presences of well resolved signals of 18 carbon atoms. The Carbon signal resonating down field at δC 174.4 designated the presence of carbonyl carbon of ester or amide group. The peaks at δC 70.3, 65.2 and 63.3 indicated typical signal for aliphatic oxygenated carbons and from these values it can be deduced that the peak at δC 174.4 will be a carbonyl carbon of ester functionality. Furthermore the DEPT-135 experiment revealed the presence of fifteen methylenes carbon atoms, of these thirteen at δC 34.2, 24.9, 29.1, 29.7, 29.7, 29.7, 29.7, 29.6, 29.5, 29.4, 29.3, 31.9 and 22.7 are aliphatic methylenes and the signals at δC 65.2 and 63.3 also inferred the presences of oxy-methylene carbons (-OCH2-and – CH2OH) that support the compound is an ester not amide. The above argument is further supported by 2D NMR experimental data (Fig. 2)
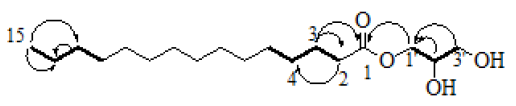
Figure 2: Key HMBC (curved arrow) and 1H-1H COSY (bold line) correlations of compound 1.
The 1H-1H correlation spectroscopy (COSY) showed strong correlation between H-2 and H-3 at δH 2.37 (2H, t, J = 7.6 Hz) and δH 1.65 (2H, q, J = 7.3 Hz) respectively. The H-3 at δH 1.65 (2H, q, J = 7.3 Hz) in turn strongly correlated with H-2 at δH 2.37 (2H, t, J = 7.6 Hz) and H-4 at δH 1.30-1.35 of (22H, m) for the overlapped protons. The triplet methylene protons at δH 2.37(2H, t, J = 7.6 Hz) showed HMBC (2J) (Fig. 2) correlations with the carbon at δC 174.2 (carbonyl carbon, C-1) and δC 24.9 (CH2, C-2) respectively. The doublet of doublet for geminal methylene protons attached to alkoxy oxygen-carbon resonate at δH 4.23 (2H, dd, J = 11.7, 4.6 Hz) on C-1'at δC 65.2(CH2) correlated (2J and 3J) with the oxygen substituted secondary and primary carbon atoms at δC 70.3(CHOH) and δC 63.3(CH2OH), respectively of the alkoxy group of ester and 3J coupled to carbonyl carbon(C-1) at δC 174.2.
Based on these spectroscopic data and comparison of the 1H NMR and 13C NMR spectral data with the literature report [15, 16], the structure of compound 1 was identified as 2', 3'- dihydroxypropyl pentadecanoate, which has previously been isolated from the rhizome of Polygonatum verticillatum.
Compound 2 was also isolated as white solid. The 1H and 13CNMR spectral data in CDCl3 (Table 1) showed similar spectral signals with compound 1 except the absence of alkoxy groups, as would be observed in 1. The proton decoupled 13C NMR (Table 1) spectrum of 2 showed well resolved signals for 15 carbon atoms. The DEPT-135 spectrum revealed the presence of one methyl, thirteen methylene groups, indicating 29 hydrogen atoms attached to 14 carbon atoms. The HMQC spectrum clearly showed the connectivity between protons at δH 2.34 (2H, t, J = 7.5 Hz) and carbon at δC 34.5 (CH2, C-2), protons at δH 1.62 (2H, m) with carbon at δC 25.1(CH2, C-3), δH 1.30 (2H, m) with carbon at δC 23.1 (1CH2, C-14) and protons at δH 0.87(3H, t, J = 6.8 Hz) with carbon at δC 14.5 (CH3, C-15) respectively. Moreover, the methylene protons at δH 2.34 (2H, t, J = 7.5 Hz) on C-2 at δC 34.5 (CH2) (methylene carbon α to carbonyl)) showed HMBC (2J) correlation with the carbons at δC 180.5 (carbonyl carbon, C-1) and β-C at δC 25.1 (CH2) respectively, as in compound 1, except with the free carboxylic acid group
The primary methyl protons at δH 0.87(3H, t, J = 6.8 Hz) correlated (2J and 3J) with the signal at δC 23.1 (CH2, C-14) and δC 32.3 (CH2, C-13) respectively. Therefore, having the information obtained from 1D and 2D NMR and comparing with the reported data [17-19], the structure 2 was deduced to be pentadecanoic acid.
Compound 3 was isolated as white solid. The 1H and 13CNMR spectra (Table 1) were virtually identical to those of compound 2, except for the difference in number of -CH2 groups. Its proton decoupled 13C NMR (Table 1) spectrum showed well resolved signals for only 14 carbon atoms and DEPT-135 spectrum also revealed the presence of one methyl, twelve methylene groups, indicating 27 hydrogen atoms attached to thirteen carbon atoms. The presence of acid carbonyl is also evident from a carbon signal at δC 179.0 (C-1). Therefore, based on this spectroscopic evidence, the structure of 3 was identified to be tetradecanoic acid, comparing with compound 2.
The isolated compounds (1, 2 and 3) were evaluated for their antibacterial activities against five bacterial strains (S. aureus (ATCC 25923), E. coli (ATCC 25922), P. aueroginosa (ATCC-27853), S. tphyimurium (ATCC 13311) and S. flexineri (ATCC 29903)) at minimum concentration of 0.5 mg/ml with standard reference gentamycin. However, no significant inhibition has been observed against the test strains. However, the crude extract showed considerable activity with zone of inhibition 10.6, 8, 15, 9.4mm against the S. aureus (ATCC 25923), E. coli (ATCC 25922), P. aueroginosa (ATCC 27853) and S. flexineri (ATCC 29903) bacterial strains, relative to standard reference gentamycin at 16, 21, 14 and 24mm respectively. This variation of inhibition may be related to the synergetic effects of the various kinds of compounds in the crude extracts or a compound responsible for the activity of the crude extract could be minor has not been isolated.
It has been reported that compound 1, isolated from rhizome of Polygonatum verticillatum showed significant inhibitory activity with IC50 value of 9.45μM against tyrosinase [16] and showed antibacterial activity against some gram-negative bacteria [20].
Phytochemical investigation of dichloromethane extract of fruits of C. ficifolius gave three compounds: 2’, 3’-dihydroxypropyl pentadecanoate (1), pentadecanoic acid (2) and tetradecanoic acid (3). This is the first report of secondary metabolites from C. ficifolius. The crude extract of C. ficifolius showed antibacterial activities against S. aureus (ATCC 25923), E. coli (ATCC 25922), P. aueroginosa (ATCC 27853) and S. flexineri (ATCC 29903), which is comparable to standard reference gentamycin. However, the isolated compounds showed no activities against these test strains.
Citation: Tamrat Ayele (2021) Chemical Constituents of the Fruits of Cucumis ficifolius and Evaluation for Antibacterial Activity. Organic Chem Curr Res. 10:8 P483.
Received: 31-Jul-2021 Accepted: 24-Aug-2021 Published: 04-Sep-2021 , DOI: 10.4172/2161-0401.21.10.483
Copyright: © 2021 Ayele T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.