Journal of Nanomedicine & Biotherapeutic Discovery
Open Access
ISSN: 2155-983X
ISSN: 2155-983X
Research Article - (2014) Volume 4, Issue 1
Water demonstrates antimicrobial properties after it is treated with room temperature non-thermal plasma. In this work, we have applied UV spectroscopy, Raman spectroscopy, Electron Spin Resonance, and Mass spectroscopy experiments to investigate chemical species in water treated with non-thermal plasma. We propose that HONOO may be the major species contributing to the antimicrobial effects of this solution. However, it is also possible that the antimicrobial effect is due to the combination of all the radicals and oxidants in the solution
Keywords: Antimicrobial solution; Indirect plasma; Nonthermal plasma; Plasma chemistry; Reactive oxygen species; Reactive nitrogen species
Medically approved disinfectants and hand washes are used routinely, many of which are not sufficient to disinfect surfaces, and fail to inactivate a substantial percent of the pathogens found within biofilms [1-3]. Strong biocides such as benzalkonium chloride, chlorhexidine gluconate, and triclosan were unable to completely inactivate pathogens in their planktonic and embedded biofilm (sessile) forms, and thus the reservoirs of hospital-acquired infections are inefficiently controlled [4,5]. There is a real need for a disinfectant solution that is less toxic, carries less fear of systemic bacterial resistance, simple, and safe for use in all patient populations including children/neonates.
Recently, several groups (including our laboratory) have discovered a potent antimicrobial solution produced by treating water or a simple non-toxic chemical solution with electrically produced room temperature non-thermal plasma [6]. Plasma is an ionized phase of matter where different charges can move separately from each other, while maintaining overall electrical neutrality on the macroscopic scale. The energetic electrons of the plasma produce excited speciesfree radicals and ions, as well as additional electrons through electronimpact dissociation, excitation and ionization of background gas molecules. In addition to electrically charged ions and electrons, it also contains large concentrations of neutral molecules and atoms. Many of these molecular species can be chemically active and live for a variable amount of time. Others can be electronically excited and will emit light as they relax to their natural state.
Thermal plasmas are in use in the medical field for various purposes, such as cauterization, in the last few decades. Recently, direct or indirect application of non-thermal, dielectric-barrier discharge (DBD) plasma, also known as cold plasma, is under investigation for its ability to disinfect and sterilize biomaterial or the surfaces likely to be damaged by thermal plasma [7]. The basic feature of non-thermal plasma is that the majority of the electrical energy used to generate the plasma primarily goes into the production of energetic electronsinstead of heating the entire gas stream. Non-thermal plasma has been employed widely for production of ozone [8], which can be used to oxidize organic molecules and kill bacteria, a realization that lead to the development of a new field-plasma medicine.
Most of the efforts in plasma medicine are focused on applying plasma directly to tissues, biological fluids and material surfaces. Recently, our laboratory and select groups discovered that reactive agents generated in water when exposed to plasma treatment that has strong antimicrobial activity, and that this property can be efficiently stored in treated water [6]. A variety of water-containing solutions have been tested so far including pure water and water with various organic compounds. Our method of application of DBD plasma, reported previously, is unique and known as floating electrode dielectric discharge-barrier (FE-DBD) plasma [9]. This discovery led us to develop this application to create an antimicrobial solution that can possibly be used to flush the contaminated surfaces of indwelling catheters to eradicate bacterial/fungal cells [10,11].
We have investigated the antimicrobial activity of plasma activated solution against the commonly isolated nosocomials as well as the pathogens responsible for catheter-related blood stream infections (CRBSI) such as E. coli, Acinetobacter baumannii, coagulase-negative Staphylococci, methicillin-resistant Staphylococcus aureus, and yeast such as Candida albicans [6]. We reported previously how direct plasma treatments inactivate contaminating pathogens when the FEDBD plasma technique is used [12]. We also demonstrated that the FE-DBD plasma technique generates ROS inside the bacterial cells on direct treatment with plasma [13]. Our recent work also suggests that the treated liquids turn into powerful antimicrobial solutions which are highly bacteriocidal against a range of pathogens, including fungi, and are as effective as the direct plasma treatment which we have reported previously [6].
We have reported that the floating-electrode technique can also deliver an antimicrobial effect (indirectly) through plasma-treated liquids or solutions and that these liquids hold their antimicrobial properties for extended periods of time [6], but the chemical composition and characterization of plasma-treated water (or solutions) are not exactly known. Recently, several groups have proposed various chemical species that are generated in water treated with plasma, but direct evidence of these species is not shown. More importantly, it has not been demonstrated whether the hypothesized solutions have appropriate antibacterial properties. Through the studies presented here, we report the determination of the chemicals in water treated by FE-DBD plasma technique by a variety of methods and propose the chemicals responsible for antibacterial properties of this solution.
Electrode and plasma
Dielectric barrier discharge (DBD) technique was used in order to generate non-thermal plasma. DBD electrode was customized by covering the surface of 38 mm×64mm copper plate with 1 mm-thick glass slide (Fischer Scientific Inc., Pittsburgh, PA) and insulating with silicone. Also, a custom designed quartz treatment chamber (referred as fluid holder), which can maintain 1 mm liquid column, was used. Discharge gap for plasma treatments was fixed at 2 mm. Characterization of plasma power generator was done in our collaborating laboratory of A. J. Drexel Plasma Institute, Drexel University according to a reported procedure [14]. One mL of deionized water (MP Biomedicals Inc., Solon, OH) was treated separately at different time points and collected immediately after plasma treatment to perform various tests.
Instrumentation for chemical analysis
A pH ultrasensitive probe attached to a Thermo Orion Research Digital pH meter (Thermo Fisher Scientific, Waltham, MA) was used to measure plasma treatment-associated pH changes in fluid over time. UV-Vis spectroscopy results were obtained from a spectrometer (SPD-20A, Shimadzu, Japan). Electron spin resonance (ESR) results were obtained from a Varian E-12 X-band EPR spectrometer. Raman vibrational studies were performed using an Renishaw RM 1000 microscope spectrophotometer using a HeCd laser wavelength of 442 nm. The MS data of the sample were acquired by using a Waters highresolution mass spectrometer (Micromass AutoSpec Ultima-Q) with EI, CI, ESI and FAB ion sources available. In this work, all ion sources were utilized. The Raman results were obtained from a confocal Raman microscope spectrometer (RM 1000, Renishaw).
Isolates and culturing of bacterial pathogens
Escherichia coli (ATCC25922) were purchased from American Type Culture Collection (ATCC, Manassas, VA). The strain was maintained and used as overnight culture in trypticase soy broth (TSB) for primary inoculations according to the supplier’s guidelines. Three percent of hydrogen peroxide (Sigma) was used as the known biocidal agent and untreated deionized water was used as a negative control.
Plasma fluid-mediated bactericidal activity
A given bacterial strain was cultured from stock TSA plate that is kept at +4°C no more than 1 month. A single isolated colony was collected with a sterile inoculation loop and inoculated into 10 ml TSB medium, and incubated at 37°C for overnight. The next day the culture was re-inoculated (10 ul into 10 ml TSB), incubated for 4 h and the optical density at 600 nm (OD600) was adjusted to 0.2 (to maintain uniform number cells consistently). The culture dilution thus prepared (1:100; that corresponded to ~ 1×107 CFU/ml during colony count assay) was mixed with plasma-treated water (50 μl:50 μl) and held together at room temperature for 15-min (predetermined contact time) After 15 minutes exposure of bacteria to plasma treated water, serial dilutions were made in 1X sterile PBS and 100 μl of diluted suspension spread on trypticase soy agar (TSA) plates to incubate at 37°C for 24 h. After incubation of plates, colony forming units (CFUs) were counted to correlate with bactericidal efficacy of plasma-treated water. The plates were continued to incubate for 72 h and visually inspected for the appearance of growth of colonies, to rule out delayed or dormant growth of bacteria. Hydrogen peroxide (3%) was used as positive control in parallel experiments.
Data analysis
All experiments had built-in negative and positive controls as stated. Wherever needed, Prism software v4.03 for Windows (Graphpad, San Diego, CA) was used for statistical analysis. A p value was derived using pair comparisons between two bacterial groups with a student’s t test and one-way analysis of variance for multiple comparisons. A p value of <0.05 was considered statistically significant. All experiments were performed a minimum of three times in triplicate, unless specifically stated.
The most notable observation of plasma-treated water is that the pH decreased from neutral to acidic (Figure 1) and remained stable during storage. We confirmed that the pH drops as the treatment time advances, up to 3 min (thereafter no change up to 5 min; which may be saturation point). At 3 min of exposure to plasma, de-ionized water showed a pH of 2.16. The most likely chemicals that contribute to acidification of treated fluid could be nitric acid and nitrous acid that are generated from NO2 and NO in the plasma, respectively. Similar changes are reported in the gliding arc-mediated plasma technique and indirect air dielectric barrier discharge [15].
Existence of both HNO3 and HNO2 were confirmed from the UV spectra (Figure 2). A group of 5 peaks in the range of 330-390 is a characteristic profile of HNO2 [16]. The peak at 302 nm was mainly attributed to HNO3, which will be further discussed later. Traylor et al. [15] reported that on initial treatment with plasma, both HNO2 and HNO3 were generated from deionized water [15]. After storage for one day to seven days, the concentration of HNO2 and HNO3 gradually deceased and increased, respectively. Our work showed a similar phenomenon-the concentration of HNO3 increased while HNO2 decreased upon longer exposure to plasma (Figure 2). Our results showed that after plasma treatment, the HNO2 disappeared much quicker within 2 min, rather than days under storage conditions. The quick disappearance of HNO2 was not mainly due to low pH since the disappearance process was slow in low pH at 2.7 [15]. All these results suggested that the plasma treated water had a significantly strong oxidative characteristic, which will be discussed later for their antimicrobial effect. The concentrations of HNO3 and HNO2 in water after 30-second treatment (Figure 2) were 0.36 mM and 0.067 mM, respectively, according to Beer-Lambert Law, a quantitative way to determine concentrations of an absorbing species in solution,
a = εbc, (1) 3.86
where a is the measured absorbance, b the path length through the sample (1 cm), and c the concentration of the absorbing species. For each species and wavelength, ε is a constant known as the molar absorptivity or extinction coefficient. The extinction coefficients of HNO3 and HNO2 are 70 M-1 cm-1 at 300 nm and 23 at 354 M-1 cm-1 nm, respectively [17]. HNO2 are not detectable after 2 min treatment. Treatment of the liquid with plasma for 1,2 and 3min generated, respectively, 1.26 mM, 2.57 mM, and 3.57 mM of HNO3. The concentration of the acid(s) in the plasma treated solution (after 2 min) is 4.7 ± 0.5 mM according to titration, which corresponds to pH 2.33, if the acid is totally dissociated, i.e. a strong acid, and higher pH if the acid is a weak acid. The pH was in line with our observed result and confirmed that the major acid in the solution that contributed to low pH is HNO3 and ruled out the major contribution from other acids, such as hydroperoxyl (HO2) and peroxynitrous acid (HONOO) although they also have absorption around 300 nm. However, these weak acids may exist in small quantities as discussed later. H2O2 is dissolved in water from the plasma. The concentrations of H2O2 are 0.42 mM, 1.66 mM, and 0.91 mM, respectively, after plasma treatments for 1, 2, and 3 min according our previous methods [6].
Our study demonstrated that deionized water treated by nonthermal DBD plasma shows excellent antimicrobial effects wherein a 7-log reduction is obtained by water which had undergone 3 minutes of plasma treatment and was given 15 minutes of holding time (contact time) with planktonic form of E. coli. Up to 2 minutes of plasma treatment caused about 1 log reduction (Figure 3). This trend is in agreement to our earlier findings [13,18]. These results suggest that the concentration of reactive chemical species that are responsible for antimicrobial effect increased and reached a concentration high enough to kill most of the bacteria when water was treated with plasma for 3 min. It also suggests that HNO2 is not the major component that contributes to the antimicrobial effect of the plasma-treated water because HNO2 appeared in the first half min and disappeared after 1 min according to the UV spectroscopy results.
In order to determine whether acid production was responsible for the strong antimicrobial effect, in a separate set of experiments, we exposed bacterial suspensions of known cell densities to 3.57 M HNO3 for exposure times equivalent to those of the plasma-treated liquid; we performed the colony assay to quantify the amount of bacterial inactivation. After a holding time, colony assays were carried out to evaluate inactivation of the pathogen. We exposed E. coli (1×107 CFU/ mL) to an equal molar concentration of nitric acid alone. The colony assay demonstrated near zero inactivation. We added 0.067 M HNO2 and 0.091 mM H2O2, which are the maximum amount observed in our experiments, to the HNO3 solution and found a minimal inactivation. The acids do not have a significant contribution to the antibacterial property of the plasma-treated water. H2O2 is a known antimicrobial agent. However, a concentration of H2O2 as low as 124 mM (0.38%) is needed for complete inactivation. A mixture of 0.91 mM (the highest amount of H2O2 in our sample), 0.067 mM HNO2, and 3.57 mM HNO3 did not inactive E. coli cells (Figure 4). These results suggest that other species in the solution play the major role in the antimicrobial properties of the treated water.
Ikawa et al. [19] concluded in their recent work that HO2/O2- might be the major chemical species in plasma-treated water that contributes to the antibacterial characteristics [19]. To determine which chemical species or which combination of chemicals species in the plasma-treated water were most likely contributing to the antimicrobial property of the solution, we further performed a series of experiments.
The pKa of HO2 is 4.88. Since the solution is highly acidic, such as pH 2.0 after 3 min exposure to plasma, HO2/O2- should be primarily in the acid form, HO2, which is the focus in this study. The maximum absorption of HO2 is at 230 nm and the extinction coefficient of HO2 at 230 nm is 1400 M-1 cm-1 [20]. In this work, we could not observe HO2 from the UV spectra, which may due to its overlap with strong peaks of HNO2 and HNO2 in 200-260 area. To further prove whether HO2 exist in the solution, we tested the solution with Raman spectroscopy and mass spectroscopy; no detectable HO2 signals were observed from these experiments (figures not shown). From these results, we tend to believe that HO2 was not the species responsible for the antimicrobial property of the solution because HO2 either does not exist in the solution, or if it does, it exists at a very low concentration [21].
The concentration of HO2 is very low because it reacts with radicals rapidly and the chemistry related to its reaction is well known as shown in reactions 1 and 2.
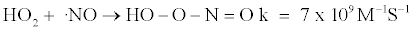 (1)
(1)
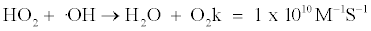 (2)
(2)
The mechanism and rate constants were obtained from two references [22,23]. Peroxynitrite is a strong and relatively long-lived oxidant [23]. However, HOONO is not stable in acid condition. HOONO dissociate in two ways, one to HNO3 at a very slow rate of 1 M-1S-1, and another to ⋅NO2 and ⋅OH at faster rates. Typically more than 90% dissociates to HNO3, but it is expected that this route is significantly diminished in the presence of a high HNO3 concentration due to le Chatelier’s principle, so HOONO may be when the HNO3 concentration is high.
The pKa of HONOO is 6.8 for the cis conformation, the more stable isomer, while the trans isomer has a pKa = 8.0. Similar to that of HO2, since the solution is highly acidic, HONOO/-ONOO should exist mainly in their acid forms, HONOO. The absorption of HONOO has never been reported although it had been suggested that the maximum absorption of absorption of HONOO is at around 202 nm [24]. However, since HNO3 has a strong absorption at 200 nm, it is not possible to distinguish the peak of HONOO from HNO3 at around 200 nm. Furthermore, we could not identify HONOO from mass spectrometry experiments, again because the overlap of its signals with those of HNO3. Furthermore, we were unable to deconvolute these peaks from Raman spectroscopy, which may due to 1) the overlap of its signals with those of strong broadened peaks of H2O2 and H2O; 2) the concentration of HOONO was lower than the detection limit by Raman spectroscopy [24,25]. It is noteworthy that we did not observe peaks of another cousin of HOONO, peroxynitric acid (HOONOO) [26].
However, although we could not identify ONOOH, we could not rule out the contribution of HOONO for the antimicrobial property of the solution. ESR results provided an evidence of the existence of HOONO. Figure 5 is the ESR spectra of freshly-treated water (3 min exposure to plasma) and the same solution after 3 days of storage in sealed container. The ESR spectra showed the possible existence of peroxynitrite radical [27]. Also this signal might be contributed to other radicals as well, this signal could not be contributed to superoxide due to the fact that superoxide typically has multiple ESR peaks [28].
In general, the term peroxynitrite is used to refer to the sum of ONOO- and its conjugated acid HOONO [29]. Without scavengers, chelators, metal ion centers, or other species present, they will have long enough lifetimes. HONOO can oxidize, nitrate, and hydroxylate biomolecules under physiological conditions, which is the origin of peroxynitrite cytotoxicity. Su et al. [30] reported the permeability coefficient for peroxynitrite was 8.0×10-4 cm/s, which is similar to that of H2O and is approximately 400 times greater than that of superoxide. This high permeability makes peroxynitrite an extremely effective oxidant in executing damage to bacteria [30,31]. Oxidation of thiols, sulfides, transition-metal centers, ascorbate, olefins, benzene, phenols, and other aromatics by peroxynitrite in vitro had been demonstrated [32]. Also, peroxynitrite exhibits a strong antimicrobial property at low concentration [33]. It is generally believed that peroxynitrite is stable only in alkaline conditions; however, based on all the results we observed, we tend to believe that peroxynitrite is stable in the unique low pH environment generated in the plasma-treated water, and it is likely the origin of the antimicrobial property of the plasma-treated water. The reason should be further studied in the future.
In conclusion, UV spectroscopy, Raman spectroscopy, ESR, Mass spectroscopy experiments showed that HONOO radical in FE-DBD non-thermal plasma-treated fluids may be the species contributing to the antimicrobial effects of the solution for longer periods.