Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
+44 1478 350008
ISSN: 2161-0398
+44 1478 350008
Research Article - (2017) Volume 7, Issue 6
Snail shells had been utilized to prepare chitosan and hydrogels of the chitosan were also prepared and crosslinked with varying amounts of glutaraldehyde to achieve different crosslink densities between 0.75 and 1.50. The materials were characterized in terms of the dependence of their swellabilities on time and pH. FTIR analysis was also carried out on the hydrogels and the results obtained show a band at 3451 cm-1, attributed to O-H stretching of the chitosan. The crosslinked hydrogels also showed an N-H bending vibration at 1635 cm-1 which has a reduced intensity and has moved to a lower wavenumber when compared to the N-H bending vibration of the uncrosslinked chitosan hydrogels at 1652 cm-1. The swelling studies showed that the extent of swelling of the hydrogels was dependent on the crosslink density (CD), increasing as CD increased. Uncrosslinked chitosan hydrogel had maximum swelling of 162.71% while that for the crosslinked chitosan hydrogels with CD of 0.75, 1.00 and 1.50 were 119.87%, 93.21% and 87.65% respectively. In all cases, their crosslinked counterparts had decreased swellabilities suggesting that, the crosslinked chitosan hydrogels can be used for a more controlled delivery of drugs and as efficient materials for tissue engineering. The chitosan hydrogels showed maximum percent swellability in highly acidic medium (pH2) equally suggesting the potential of these hydrogels as drug release systems in this medium. The swelling of the chitosan hydrogels followed second-order kinetics and their swelling diffusion exponents ranged from 0.142 to 0.155, indicative of a Less Fickian diffusion or transport mode.
<Keywords: Chitosan; Hydrogels; Swellability; Drug delivery; Tissue engineering
A hydrogel (also called aquagel) is a three-dimensional macromolecular network of polymer chains that are hydrophilic, sometimes found as colloidal gels in which water is the dispersion medium. Three-dimensional networks are usually formed by chemical or physical crosslinking of hydrophilic polymer chains. In chemical gels, polymer chains are connected by covalent bonds, but in physical gels they are held together by noncovalent bonds, such as van der Waals interactions, ionic interactions, hydrogen bonding, hydrophobic interactions, traces of crystallinity and multiple helices or by molecular entanglements. Different synthetic, natural and modified natural polymers, including chitosan, are used to form hydrogels [1]. Hydrogels are stable upon swelling in water and are capable of absorbing a large amount of water, varying from 10% to thousands of times of its own volume. Hydrogels based on natural polymers are currently receiving a great deal of interest, and are notable for controlled delivery of bioactive molecules and tissue engineering [2].
The hydrogels of chitosan, like other hydrogels, contain much water. Part of this water is tightly bound to the polymer and the rest is present as free water [3]. Chitosan-based hydrogels have been reported to exhibit good biocompatibility, low degradation and processing ease. The ability of these hydrogels to swell in water and dehydrate depends on its composition, biodegradability, and environment. These dependences have been exploited to facilitate a range of applications such as drug release [3]. Chitosan has also been defined as the deacetylated derivative of chitin which is a water insoluble polymer (N-acetyl-D-glucosamine), found in nature as present in the outer shells of snails, crabs, shrimps, lobsters, insects, and fungal cell walls. It is a natural, biodegradable, biocompatible, bioadhesive polymer, and is gaining attention in the pharmaceutical field for a wide range of drug delivery [4] and in tissue engineering [5]. Chitosan is also a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine, which is the acetylated unit [4]. Chitosan has a rich history of being researched for applications in medicine, agriculture, biology, and horticulture dating back to the 1980s. However, chitosan has certain limitations for use in controlled drug delivery and tissue engineering. These limitations can be overcome by chemical modification. Thus, modified chitosan hydrogels by crosslinking have gained importance in current research on drug delivery and tissue engineering systems [2]. Modifications of chitosan can improve the polymers’ inherent properties which include biocompatibility, chemical versatility, biodegradability and low toxicity. These modifications can be tailored for a specific application [6]. For example, crosslinking chitosan with crosslinking agents such as glutaraldehyde or sodium tripolyphosphate, has proved to be a convenient and effective method of improving the physical and chemical properties of chitosan for practical applications [7].
The wide varieties of applications of chitosan are due not only to its excellent biocompatibility, biodegradability, and economic efficiency, but also due to its distinct chemical structure with high percentage of primary amino groups (-NH2) for easy binding to biomolecules such as DNAs and proteins [5].
Drug release from chitosan-based solid dosages depends upon the morphology, size, density, and extent of crosslinking of the particulate system, physico-chemical properties of drug as well as the polymer characteristics such as whether the polymer is hydrophilic or hydrophobic, has gel formation potentials, swelling capacity, mucoor bio-adhesive properties and also depends on the presence of other excepients present in the dosage form [8]. Since chitosan does not cause any biological hazard and is inexpensive, it is suitable for use in the preparation of solid dosage forms of commercial drugs [7] and as materials for tissue engineering [5].
Materials
Glutaraldehyde (pentane-1,5-dial), was supplied by Qualikems (India). Sodium hydroxide, acetic acid, tween-80, sodium hypochlorite, and concentrated hydrochloric acid were of analytical grades from BDH.
Preparation of chitosan from snail shells
African giant land snail shells (Archarchatina marginata) were collected from a local market in Mbaitoli Local Government Area of Imo State, Nigeria, washed, sun-dried for two weeks and pulverized. The ground shells were later sieved with a mesh sieve (425 μm). The sieved snail shell powder (250.0 g) was treated with 3.0 L of 1.2 M NaOH solution for two and half hours at 75°C, with stirring at intervals. After the heating process, the solution was allowed to cool, then, the excess NaOH solution was removed by decantation, followed by washing with deionized water to neutral pH, filtration, and air-drying of the residue. The recovered sample from the deproteinization process was placed into 2.72 L of 0.7 M HCl solution for 20 minutes. The excess HCl solution was removed by decantation, followed by washing of the sample to neutral pH with deionized water, filteration and air-drying. The sample obtained from the decalcification process was dispersed in 1.5 L of 0.3% (v/v) solution of NaOCl (containing 12.5% available chlorine). The mixture was allowed to stand for 1 hour and the excess NaOCl removed by decantation, followed by washing to neutral pH with deionized water and air drying to obtain chitin. The chitin was treated with 1.4 L of 50% NaOH solution (i.e., 12.5 M) for 20 minutes at 120°C. The solution was allowed to cool, then, the excess NaOH solution was removed by decantation, followed by washing of the sample to neutral pH, filteration with a sintered glass and air drying to obtain chitosan.
Preparation of chitosan hydrogels
Preparation of uncrosslinked chitosan hydrogel: 1.5 g of chitosan was dissolved in 20.0 mL of 2.0% aqueous acetic acid at room temperature with continuous stirring for 24 hours to obtain a pale yellow viscous chitosan solution. Few drops of 0.5% tween-80 were added to the solution for uniform dispersion and to prevent aggregation at ambient temperature during stirring. The chitosan solution was filtered with a sintered glass crucible to remove any undissolved matter. The viscous solution was cast into petri dishes and dried overnight at room temperature to obtain the hydrogels. The semi-dried hydrogels were further dried in an oven at 45°C for 12 hours to completely remove the residual solvent.
Preparation of crosslinked chitosan hydrogel: In a typical experiment, three separate 1.5 g of chitosan were dissolved in 20.0 mL of 2.0% aqueous acetic acid in three different beakers at room temperature with continuous stirring for 24 hours to obtain pale yellow viscous chitosan solutions. Few drops of 0.5% tween-80 were again added to the solutions. The solutions were also filtered with the sintered glass crucible and 0.1% aqueous glutaraldehyde solution in different amounts (1.0 mL, 1.5 mL, and 2.0 mL) were added to the three different samples of clear pale-yellow chitosan solutions to obtain solutions with different crosslink densities of 1.50, 1.00, and 0.75 respectively. The solutions were stirred for 30 minutes at room temperature as they became increasingly viscous and with more intense colour. These solutions were thereafter cast into a petri dishes and dried overnight at room temperature to form the crosslinked chitosan hydrogels. The semi-dried, crosslinked hydrogels were further dried in an oven at 45°C for 12 hours to completely remove the residual solvent.
Characterization of the hydrogels
Structural characterization of the hydrogels by Fourier Transform Infrared (FTIR) spectral analysis: Infrared transmission spectra of the hydrogels were studied using Perkin-Elmer FTIR spectrophotometer (model 2000) in KBr discs from 4000 to 400 cm-1. This FTIR study was carried out to the functional groups in the hydrogels.
Swellability studies: The swelling ability of the uncrosslinked and crosslinked chitosan hydrogels were measured by determining the percentage of swelling (S) using Equation (1).
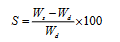 (1)
(1)
where Wd is the mass of dry sample in g and Ws is the mass of swollen sample at time t. Completely dry hydrogel of known mass (Wd) was immersed on definite time period in buffer solution and after time t, the sample was taken out from the solution and quickly wiped with filter paper and weighed (Ws). The swellability studies of the test materials were carried out in water (27°C) at different pH values (2, 4, 6, and 8) and the effect of time on the percent swellability was also carried out at time intervals of 10, 20, 30, 40, 50, and 60 minutes, at a fixed pH of 2.
Swelling kinetic experiment: The swelling kinetic experiment was carried out by measuring the weights of the hydrogels up to the equilibrium swelling state. Dry hydrogels of known weigths were kept in water at 27°C for 3 hours to allow them swell up to equilibrium, using the method of Bamgbose et al. The S at equilibrium (Seq) can be got using Equation (2).
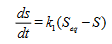 (2)
(2)
where Weq is the swollen weight of hydrogels at equilibrium.
Result of FTIR analysis of the uncrosslinked and crosslinked chitosan hydrogels
The FTIR spectrum of uncrossslinked chitosan hydrogel presented in Figure 1 shows a broad band at 3451 cm-1, attributed to O-H stretching, which overlaps the N-H stretching in the same region. The band at 2931 cm-1 represents -CH2 aliphatic groups. The band at 1652 cm-1 is the N-H bending vibration. The bands at 1461 cm-1 and 1384 cm-1 are the C-H bending vibrations of alkyl and methyl groups respectively. The methyl group may have come from the acetic acid used as solvent in the preparation of the chitosan hydrogel. The bands at 1167 cm-1 and 1045 cm-1 are attributed to -C-O-C- glycosidic linkage in the chitosan ring.
The FTIR spectrum of the crosslinked chitosan hydrogel presented in Figure 2, shows bands at 3417 cm-1, O-H stretching which overlaps the N-H stretching in the same region, 2925 cm-1 and 2365 cm-1 attributed to the -CH2 aliphatic groups, 1461 cm-1 and 1384 cm-1 represent the C-H bending vibrations of alkyl and methyl groups respectively, 1167 cm-1 and 1028 cm-1 which are attributed to the -C-O-C- glycosidic linkage, and an N-H bending vibration at 1635 cm-1 which has a reduced intensity and has moved to a lower wavenumber when compared with the N-H bending vibration of uncrosslinked chitosan hydrogel at 1652 cm-1. This can be attributed to the effective crosslinking of chitosan hydrogel with glutaraldehyde, which occurred at the amino groups of chitosan.
Results of the swellability test on the chitosan hydrogels
The appearance of the cast film of a chitosan hydrogel showing homogeneity is presented in Figure 3.
Effect of time: The results of the percent swellability studies at varying time intervals of the uncrosslinked and glutaraldehydecrosslinked chitosan hydrogels with different crosslink densities are as presented in Figure 4. The results represented in Figure 4 show that the percent swellability of the uncrosslinked chitosan hydrogel increases as time increases. This is because of the hydrophilic nature of chitosan, which is as a result of the presence of -OH groups in chitosan and the flexible nature of the matrix. Additionally, the percent swellability of the crosslinked hydrogels increases as their crosslink densities increase but the extent is lower than that for the uncrosslinked chitosan hydrogel. In the first 10 minutes, the percent swellability was about 70% for the uncrosslinked hydrogel and between 35% and 40% depending on the crosslink density for the crosslinked chitosan hydrogels. This is because, crosslinking of the chitosan with glutaraldehyde occurs at the amino groups (-NH2) of the chitosan and this is aided by the lone pair of electrons on the nitrogen atoms of the amino groups, resulting in the nucleophilic attack of the nitrogen atoms to the carbonyl carbons of glutaraldehyde. As a result of this, there is reduction in water absorption sites in the crosslinked hydrogels. This explains why the uncrosslinked chitosan hydrogel exhibits a higher swellability with time, when compared with the crosslinked hydrogels.
The mechanism for the reaction between the amino groups of chitosan and the carbonyl groups of glutaraldehyde as summarized by [9] is shown in Figure 5.
In general, the swellability of chitosan and its hydrogel are affected by three factors:
1. Presence of hydroxyl (-OH) groups in the chitosan chain, which enhance their hydrophilicity.
2. Presence of amino (-NH2) groups in the chitosan chain, which get protonated in water, mostly in acidic medium.
3. Flexibility of the chitosan polymeric matrix, which can allow for easy penetration of the solution [10].
Effect of pH: The effect of pH on the percent swelability of uncrosslinked and crosslinked chitosan hydrogels is as represented in Figure 6. The results in Figure 6 for the dependence of percent swellability on pH shows that all the chitosan hydrogels exhibited high swellability at low pH values of 2 to 4, with a rapid decline at pH 8. The uncrosslinked chitosan hydrogel swelled more than the crosslinked hydrogels at all the pH values of 2 up to 8. Generally, the percent swellability of the crosslinked hydrogels increases as crosslink density increases at pH 2 and pH 4 in the order of crosslink density 1.50>1.00>0.75 but decreases at pH 6 and pH 8.
At low pH, protonation of the amino groups of chitosan takes place, leading to repulsion in the polymer chains and the dissociation of secondary interactions such as intramolecular hydrogen bonding, allowing more water into the gel network [3]. At high pH, deprotonation of amino groups ensue, repulsion in the polymer chains is receded and this allows contraction of their polymer matrices which tends to restrict intake of water. In addition, crosslinking is known to make the gelled matrix more compact, thus, no significant water can penetrate, and as a result, there will be a reduction in swelling and an eventual collapse of the polymer matrix. These explain why the crosslinked hydrogels showed reduced swelling when compared with the uncrosslinked hydrogel.
Results of the swelling kinetic experiment
The kinetic order which the swelling of the hydrogels follows as well as their rates of swelling can be determined, according to the method described by Druzynska et al. [1].
First-order kinetics: For a first order kinetics, the swelling rate at any time can be expressed thus:
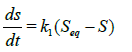 (3)
(3)
where k1 is the first order rate constant.
Integrating Equation (3) between the limits of S=0 at t=0 and S=S at t=t, we have that
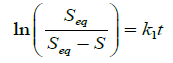 (4)
(4)
Plot of ln(Seq/(Seq-S)) against t for the uncrosslinked and crosslinked chitosan hydrogels is presented in Figure 7.
A plot of ln(Seq/(Seq-S)) against t should have given a straight line but from Figure 7, curves were observed as against straight lines, indicating that the swelling process of the hydrogels did not obey Equation (4) and as such do not follow first-order kinetics.
Second-order kinetics: The swelling rates of the hydrogels can be expressed as Equation (5) if one assumes these swelling rates follow second-oreder kinetics.
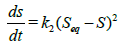 (5)
(5)
where k2 is the second-order rate constant.
Integrating Equation (5) between the limits of S=0 at t=0 and S=S at t=t, we have that
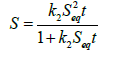 (6)
(6)
Rearranging Equation (6), we also have that
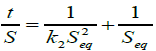 (7)
(7)
Equation (7) is the Schott’s equation.
Plots of t/S against t for the hydrogels should fit a straight line and the values of Seq and k2 can be got from the slope (1/Seq) and the intercept (1/k2Seq2) of the plots.
Plots of t/S against t for the uncrosslinked and crosslinked chitosan hydrogels is presented in Figure 8.
From Figure 8, it can be seen that our experimental data fit the Schott’s equation over the range of values used in this study. The plots gave straight lines and calculated values for Seq (Seqcalc. ) and the rate constants k2 have been got from the slopes and intercepts of the plots respectively.
The values for S, experimentally determined S at equilibrium (S), k2, and the correlation coefficients (R2) for both the uncrosslinked and crosslinked chitosan hydrogels are presented in Table 1.
| Bead Type | 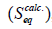 |
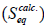 , , |
k2 × 105 (min-1) | R2 |
|---|---|---|---|---|
| Uncrosslinked | 138.420 | 142.857 | 4.900 | 0.999 |
| 1.50 CD | 64.170 | 66.667 | 22.500 | 0.996 |
| 1.00 CD | 52.310 | 55.556 | 32.400 | 0.999 |
| 0.75 CD | 48.950 | 50.000 | 40.000 | 0.999 |
Table 1: Second-order swelling kinetic parameters for the uncrosslinked and crosslinked chitosan hydrogels.
The results presented in Table 1 show that the kinetic model agreed well with the swelling experiment with R2 values of up to 0.999. This shows good correlation between t/S values and those of t. It can also be seen from Table 1 that k2 increases as CD decreases, showing that the higher the extent of crosslinking of the chitosan matrix, the more difficult it becomes for solvent to penetrate such matrices. For instance, chitosan hydrogels with CD of 1.50 had k2 × 105 value of 22.500 while the chitosan hydrogel with CD of 0.75 had 40.000. Crosslinking of the matrices lead to improved compactness of the matrices conferring such hydrogels with the ability to tightly hold incorporated drugs or genetic materials and release them at slower or more controlled rates than is expected for uncrosslinked beads.
Diffusion mechanism of water into the hydrogels
When a swellable polymeric hydrogels such as chitosan hydrogels is brought into contact with water, the water will diffuse into the polymeric hydrogel and the hydrogel expands leading to its swelling. This diffusion involves the migration of water into pre-existing or dynamically formed species between the polymeric chains of the hydrogel. The swelling of these hydrogels involves larger scale segmental motion, leading to an ultimate increase in separation of the polymeric chains of the hydrogels. The utility of the diffusion phenomena study of water into the polymeric hydrogels is to clarify the behaviour of the polymers [11].
In order to understand the type of diffusion of water into the hydrogels, the simple and commonly used Korsmeyer-Peppas model [12], based on the power-law expression got from Fick’s second law of diffusion was applied thus:
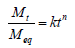 (8)
(8)
where k is a constant that depends on the morphology as well as the interaction of the polymer network, Mt and Meq are the mass uptake of water at time t and at equilibrium, respectively. The value of the swelling diffusional exponent (n) determines the transport mode or diffusion mechanism of water through the chitosan hydrogels. Equation (8) is only valid in analyzing the first 60% swelling of the hydrogels, irrespective of the geometry [13].
To determine the nature of diffusion mechanism which the diffusion of water into the chitosan hydrogels follows, the swellability data generated in this study were fitted into the linearized form of Equation (8) thus:
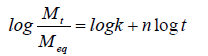 (9)
(9)
A Fickian diffusion has an n value of 0.5 (case I transport) and 0.5˂n˂1.0 for an anomalous or non-Fickian diffusion. n˂0.5 for a less Fickian diffusion (also classified as Fickian diffusion) and n=1.0, for a relaxation controlled (case II transport) release. A super case II transport which occurs occasionally, is attributed to cases where n˃1.0 [13]. Plots of log(Mt/Meq) against logt gave straight lines as presented in Figure 9 and the values of n for the hydrogels were got from the slopes of the plots. Values of n for the swelling studies of both crosslinked and uncrosslinked chitosan hydrogels are presented in Table 2. The symbol ‘NA’ represents hydrogel formulations that were not analyzed. This is due to the fact that S for such hydrogels were above 60 %, where Equation (8) is valid. The results presented in Table 2 show n values ranging from 0.142 to 0.155, indicating that the type of diffusion or transport mode of water into the hydrogels is Less Fickian (also classified as Fickian diffusion)-where water diffusion rate is much below the polymer chain relaxation rate.
| Bead Type | n |
|---|---|
| Uncrosslinked | NA |
| 1.50 CD | 0.142 |
| 1.00 CD | 0.155 |
| 0.75 CD | 0.150 |
Table 2: Swelling diffusion exponent (n) for uncrosslinked and crosslinked chitosan hydrogels.
Chitosan was prepared from snail shells and was successfully modified by chemical crosslinking with glutaraldehyde. FTIR study of the crosslinked chitosan hydrogels showed a reduced intensity of the N-H bending vibration and at a lower wavenumber when compared with the N-H bending vibration of uncrosslinked chitosan hydrogel and this was attributed to the successful crosslinking of chitosan hydrogel with glutaraldehyde, which occurred at the amino groups of chitosan. The swelling abilities of the hydrogels have been studied at different pH values and it has been found that the swelling behaviour of the hydrogels followed first-order kinetics and depended on time and the pH of the medium, decreasing as pH increases and increasing as time increased. Crosslinking of the hydrogels have also proved effective to compact the polymer blends and consequently decreasing the swelling abilities of the hydrogels. The mechanism of penetration of water into the uncrosslinked and crosslinked hydrogels was by Less-Fickian type of diffusion or transport mode. The results obtained in this study show that the chitosan hydrogels can be efficient matrices for controlled drug delivery and tissue engineering. Crosslinking of the matrices has been determined in this study to be capable of reducing swellability of the matrices, which can result in a decrease in the rate of drug delivery, with increased potential for tissue engineering.
The authors appreciate the professional advice of Prof. Arthur Jideonwo of the Department of Chemistry, University of Benin, Nigeria.