
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research - (2022)Volume 13, Issue 5
Autism Spectrum Disorders (ASD) is characterized by frequent comorbid conditions. Previously, we reported changes in circulating levels of microRNA (miRNA) determined using high throughput sequencing, which were dependent on monocyte cytokine profiles. This study assessed how levels of 7 miRNAs selected based on our previous results, change in association with both comorbid conditions and monocyte cytokine profiles. Circulating levels of miRNAs were measured by quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR) in 130 ASD and 50 non-ASD subjects. miRNA levels were negatively correlated with the production of monocyte cytokines (TNF-α, IL- 6, IL-1ß, and IL-10) in ASD subjects without sleep or seizure disorders, but not in ASD subjects with seizure/sleep disorders or in non-ASD controls. This was most evident between levels of miR-320b, miR-423-5p, miR-378-3p, and miR193a-5p, and the spontaneous production of these cytokines. ASD subjects without seizure/sleep disorders revealed higher circulating levels of these miRNAs than those with seizure/sleep disorders.
Longitudinal measurement of the miRNAs in 4 of the ASD subjects indicated an association between miRNA levels and changes in the severity of their co-morbid conditions. These circulatory miRNAs may serve as biomarkers of inflammation in ASD, given their regulatory actions on inflammation.
Autism spectrum disorders; Cytokine; Monocytes; microRNA (miRNA)
Autism Spectrum Disorder (ASD) is defined by subjective behavioral symptoms. Its complex pathogenesis is thought to be affected by a variety of genetic and environmental factors. Effects of the contributing factors vary in each ASD subject and individualized treatment measures are desirable for optimal health and developmental outcomes.
It is well known that ASD subjects exhibit various comorbid conditions that include sleep disorders, Gastrointestinal (GI) symptoms, and seizure disorders at a high frequency [1,2]. Neuroinflammation has been proposed to have a role in the pathogenesis of common neuropsychiatric conditions, including ASD, and immune mediated inflammation is also likely to have a role in the comorbid conditions that ASD subjects frequently suffer from [3- 7]. An animal model of autism, called Maternal Immune Activation (MIA), is based on sterile immune activation in pregnant rodents.
Namely, the MIA model generates ASD like behavioral symptoms in offspring by inducing sterile inflammation in pregnant rodents through injection of endotoxin intraperitoneally [8]. Behavioral symptoms observed in ASD children are often not static, frequently they worsen and there is even loss of once acquired cognitive functioning following each unrelated immune insult [9]. Such observations indicate that antigen non-specific innate immune responses likely play a role in neuro-inflammation observed in ASD subjects, as shown in the MIA model.
Our previous studies on ASD subjects revealed that a subset of ASD children did show innate immune responses skewed toward inflammation, when Purified Peripheral Blood Monocytes (PBMo) were used as representative innate immune cells [10]. Altered innate immune responses in ASD monocytes were shown to be detected at both the miRNA and the cellular function levels [11]. Such ASD subjects who revealed innate immune abnormalities often have a history of triggering immune stimuli during the mother’s pregnancy and/or in the first 2-3 years of life. Potent innate immune stimuli are known to cause prolonged effects, which in turn generate Innate Immune Memory (IIM). IIM has been shown to be generated by metabolic and epigenetic changes which cause persistent hyper or hypo-responsiveness of innate immunity (Trained Immunity (TI) vs. tolerance) [12-15]. Considering the long-lasting effects of IIM and evidence of on-going neuro-inflammation in ASD, we hypothesized that regulatory molecules such as miRNA secreted into circulation in the form of exosomal miRNAs are altered in ASD subjects. This is because exosomal miRNAs in circulation are uniquely stable and monocyte/macrophage lineage cells serve as a major source of circulating exosomal miRNAs along with platelets [16]. Functions of cells that have taken up circulating miRNAs are known to be affected, supporting the role of miRNAs as 2nd messengers. Thus, their high stability and resistance to degradation makes circulating miRNAs ideal biomarkers. In fact, circulating miRNAs have been shown to correlate with disease activity in various inflammatory and neurodegenerative conditions including Multiple Sclerosis (MS) [17,18].
We initially assessed changes in circulating miRNAs using high throughput sequencing. Our results revealed changes in several circulating miRNAs in ASD subgroups categorized based on monocyte cytokine profiles. In this study, we determined circulating levels of 7 miRNAs using qRT-PCR. These select miRNAs correlated with changes in monocyte cytokine profiles, and were not shown to be not affected by covariables including age, gender, ASD severity, and common neurotropic medications [Selective Serotonin Reuptake Inhibitors (SSRIs), Anti-Epileptic Drugs (AEDs), neuroleptics, and medications for Attention Deficiency Hyperactivity Disorders (ADHD)] [19]. In this study, we hypothesize that circulating levels of these miRNAs and their association with monocyte cytokine profiles are affected by comorbid conditions for which immune mediated inflammation plays a role in ASD subjects.
The results of this study revealed positive correlations between PCR Cts of miRNAs and production of monocyte cytokine. However, this was only found in ASD subjects without seizure or sleep disorders. No such correlations were found in non-ASD controls or ASD subjects with sleep or seizure disorders. Moreover, the circulatory levels of the 7 miRNAs are selected were generally higher (hence low PCR Cts) in the ASD subjects without sleep or seizure disorders as compared to non-ASD controls or ASD subjects with sleep and/or seizure disorders. Interestingly, in ASD subjects whose serum samples were obtained at multiple time points, we found that the circulating levels of these miRNAs appeared to correlate with changes in severity of their comorbid conditions. The presented results may support the feasibility of using these circulatory 7 miRNAs as biomarkers for chronic inflammation (possibly neuroinflammation) in ASD.
A study subjects
This study was reviewed and approved by our institutional review board: the protocol 19:53 (approval on 12/19/2019) at our institution (Saint Peter’s University Hospital, New Brunswick, NJ, United States). The study protocol includes both ASD and neurotypical control subjects. The signed consent forms were obtained prior to sample obtainment. Parents or guardians signed the consent forms when the study subjects were minors (<18 years of age) or were unable to give consent by him/herself due to intellectual disability. We assessed comorbid conditions including Food Allergy (FA), asthma, Allergic Rhinitis (AR), Specific Antibody Deficiency (SAD), sleep disorder, and seizure disorders in ASD children through direct interview and chart review. It is of note that evaluations for allergy, asthma, and SAD were often done in our clinic; the correspondence author sees many ASD subjects for evaluation of allergy and immunological disorders. Subjects with chromosomal abnormalities, well defined gene mutations, or chronic diseases involving major organs were excluded from this study. However, individuals with minor medical conditions that are present at high frequency in the general population were not excluded. These conditions included seasonal allergy, asthma, and eczema.
ASD subjects: We recruited a total of 130 ASD subject from the Pediatric Allergy/Immunology Clinic. ASD diagnosis was based on evaluation from various autism diagnostic centers, including ours. The Autism Diagnostic Observation Scale (ADOS) and/ or Autism Diagnostic Interview-Revisited (ADI-R), and other standard measures were used for diagnosis. Behavioral symptoms and sleep habits were assessed using with the Aberrant Behavior Checklist (ABC) and the Children’s Sleep Habits Questionnaires (CSHQ), respectively, provided the parents agreed to fill out the questionnaires [20,21]. Cognitive ability and adaptive skills were obtained from school records, provided the evaluations were conducted within 1 year from the time of entering the study. Evaluations by school generally included standard measures such as the Woodcock-Johnson III test (for cognitive functioning), and the Vineland Adaptive Behavior Scale (for adaptive skills) [22].
Non-ASD controls
We recruited a total of 50 non-ASD subjects as controls from the Pediatrics Subspecialty and General Pediatrics Clinics at our institution. These subjects were neurotypical and did not have any conditions listed in the exclusion criteria. Demographics of the study subjects (Table 1).
| ASD1 subjects (N=130) | Non-ASD controls (N=50) | |
|---|---|---|
| Age (years) 2 Average ± SD Median, range | 12.5 ± 5.9 12.0 (3.0-26.3) |
12.9 ± 6.2 12.3 (3.0-29.0) |
| Gender (M:F) | 113:17 (86.9%: 13.1%) | 40:10 (80.0%: 20.0%) |
| Ethnicity | AA 3, Asian 34, Mixed 5, W 102 | AA 1, Asian 5, Mixed 1, W 43 |
1Abbreviations used: AA; African American, ASD; autism spectrum
disorder, F; female, M; male, SD; standard deviation, W; Caucasian.
2Age when enrolled to the study.
Table 1: Demographics of ASD and non-ASD study subjects.
The frequency of comorbid conditions was assessed based on medical records and laboratory findings. Diagnosis of allergy and immunological conditions were diagnosed as follows:
Diagnosis of Food Allergy (FA)
Diagnosis of IgE mediated FA was based on clinically documented reactions to offending food, occurring within 2 hours after intake, and positive reactivity to Prick Skin Test (PST) and/or positive serum IgE antibody against offending food. Diagnosis of Non-IgE mediated FA (NFA) was made upon resolution of GI symptoms within 2-3 weeks following avoidance of offending food and recurrence of symptoms following challenge of offending food in the absence of PST reactivity or FA specific serum IgE [23].
Diagnosis of asthma and AR
Diagnoses of AR and Allergic Conjunctivitis (AC) were based on positive PST reactivity and/or positive serum IgE specific to aeroallergens in the presence of corresponding clinical features [24,25]. Asthma diagnosis is based on the asthma guidelines from the Expert Panel Report 3 [26].
Antibody deficiency syndrome
Specific Antibody Deficiency (SAD) was diagnosed if the subject failed to produce protective levels of antibodies against >70% of serotypes of Streptococcus pneumonia following the booster dose of Pneumovax® or PCV13®.
Pneumococcal antibody (Ab) levels that were greater than 1.3 μg/ ml were defined to be protective [27].
The frequencies of comorbid conditions in the study subjects are summarized in Table 2.
| Comorbid conditions | ASD subjects (N=130) | Controls (N=50) |
|---|---|---|
| GI1 symptoms | 81/130 (62.3%) | 0 |
| Seizure disorders | 19/130 (14.6%) | 0 |
| Asthma | 8/130 (6.2%) | 1/50 (2.0%) |
| Allergic rhinitis | 23/130 (17.7%) | 6/50 (12.0%) |
| Specific antibody deficiency | 24/130 (18.5%) | 0 |
| PANS like symptoms | 93/130 (71.5%) | 0 |
| Disturbed Sleep | 49/130 (37.7%) | 0 |
1Abbreviations used: ASD, autism spectrum disorder, GI, gastrointestinal, PANS, pediatric acute onset neuropsychiatric syndrome.
Table 2: Frequency of comorbid conditions in ASD and non-ASD subjects.
Sample collection
Blood samples were obtained via venipuncture by the physician. For most subjects, only one blood sample was obtained. For 8 ASD subjects, we obtained two blood samples to assess the variability of circulating levels of miRNAs. Four ASD subjects with varying behavioral symptoms had samples taken at 3 to 4 time points. A topical lidocaine/prilocaine cream (Emla cream®) was applied to the site of venipuncture 45-60 min prior to the procedure, if requested by parents or the study subjects.
Cell cultures
Peripheral Blood Mononuclear Cells (PBMCs) were separated with the use of Ficoll-Hypaque density gradient centrifugation. Then PBMos were separated by passing PBMCs through a column of magnetic beads labeled with anti-CD3, CD7, CD16, CD19, CD56, CD123, and glycophorin A (monocyte separation kit II–human, MILTENYI BIOTEC, Cambridge, MA, United States). In this way, monocytes were negatively selected by deleting T, B, natural killer, and dendritic cells from PBMCs.
Purified PBMos were incubated overnight (2.5 × 105 cells/ml) with the use of stimuli, as described below, and the culture supernatant was harvested for cytokine assays.
Lipopolysaccharide (LPS), a TLR4 agonist, was used as a representative stimulus for a signaling pathway activated by gram negative [G (-)] bacteria. Zymosan, a TLR2/6 agonist that mimics activation signals in response to G (+) bacteria and fungi was also used. CL097, a TLR7/8 agonist, was also utilized to represent innate signaling pathways activated by ssRNA viruses, common causes of seasonal respiratory infection. We also used candida heat extract as a source of ß-glucan, a representative C-lectin receptor agonist. Purified monocytes were incubated with LPS (0.1 μg/ ml, GIBCO-BRL, Gaithersburg, MD, USA), zymosan (50 μg/ml, Sigma-Aldrich, St. Luis, Mo), C097 (water-soluble derivative of imidazoquinolinone, 20 μM, InvivoGen, San Diego, CA, USA), candida heat extract (HCKA, heat killed candida albicans (107 cells/ml, InVivogen, San Diego, CA), and candida heat extract plus LPS in RPMI 1640 with additives [28].
Enzyme-Linked Immuno-Sorbent Assays (ELISA) were used for measuring levels of C-C chemokine ligand 2 (CCL2), interleukin (IL)-1β, IL-6, IL-10, IL-12p40, IL-23, Transforming Growth Factor-β (TGF-β), Tumor Necrosis Factor-α (TNF-α), and soluble TNF Receptor II (sTNFRII)] with the use of 10-100 μl culture supernatants/well. The OptEIA™ Reagent Sets (BD Biosciences, San Jose, CA, USA) were used for ELISA of IL-1ß, IL-6, IL-10, IL- 12p40, and TNF-α. Reagents obtained from BD Biosciences and R & D (Minneapolis, MN, USA0 were used for ELISA of CCL2, sTNFRII, and TGF-β. IL-23 levels were measured with the use of an ELISA kit from eBiosciences, San Diego, CA.
Measurement of circulating miRNA in the serum
The miRNAs were prepared from frozen serum samples with the use of a miRNA purification kit (Norgen Bioteck Corp., Thorold, ON, Canada) which uses no phenol during the purification process. This methodology was used for high throughput sequencing of circulating miRNAs in our previous study with good yield. Seven miRNAs were selected based on the results of our previous study: these miRNAs revealed significant differences among ASD subgroups and were not affected by the co-variables [29]. miRNAs were quantified by qRT-PCRs through Norgen Biotek Corp per fee service. Namely miRNA quantification was conducted with LNA based PCR and 5S ribosomal RNA (rRNA) was used as a control house-keeping RNA target, following protocols from the miRCURY LNA
PCR-Exosome, serum/plasma and other biofluid samples handbook (Quiagen Germantown, MD). miRCURY LNA RT kit and miRCURY LNA SYBR Green PCR kit were used for this process. PCR conditions were 95°C for 2 min, 40 cycles of 95°C, and 10s, and 56°C for 60s, followed by melting curve analysis at 60- 95°C. All measurements were done in duplicate. qRT-PCR results were normalized by subtracting a mean PCR Ct of 5S rRNA from a mean PCR Ct of miRNAs.
Statistical analysis
For assessing the differences of two groups of numerical data, a two tailed Mann Whitney test was used, with concurrent assessment of distribution with the use of the Kolmogorov Smirnov test. Differences in multiple numerical data sets were assessed with the use of one-way ANOVA, with concurrent assessment of normality using multiple measures. A Spearman test was used for determining a linear correlation between two numerical data sets. The results of the Spearman test were verified by the Kendall tau b test. We considered p value of less than 0.05 as nominally significant. Covariance analysis was conducted with the use of one-way ANOVA. For all statistical analysis, we used NCSS21 software (NCSS, LLC. Kaysville, UT).
Associations between the monocyte cytokine production and circulating levels of the 7 miRNA measured
We assessed whether there were any associations between monocyte cytokine profiles and the circulating levels of the 7 miRNAs selected, and if so, whether they were affected by clinical co-variables. The results are summarized in Tables 3 and Supplementary Table 1. Nominally significant associations between monocyte cytokine production (TNF-α, IL-6, IL-1β, and IL-10) and PCR Cts of miRNAs were found in ASD subjects, most notably with spontaneous production of these cytokines as shown in Table 3. Significant associations were found between miRNA PCR Cts and monocyte cytokine production under other culture conditions as well (Supplementary Table 1). In ASD subjects, PCR Cts of miRNAs were mostly positively associated with monocyte cytokine production (Tables 3 and Supplementary Table 1). However, IL- 1β and IL-6 production under cultures stimulated with CL097 or β-glucan plus LPS revealed negative associtions (Table 4 and Supplementary Table 1). In contrast, non-ASD control subjects did not reveal any associations except for IL-1β and IL-12 production (Table 4). That is, in non-ASD subjects, miRNA PCR Cts were negatively associated with IL-1β or IL-12 production (Table 4).
| ASD (N=130) | miR-193a-5p1 | miR-379-5p | miR-134-5p | miR-382-5p | miR-378a-3p | miR-423-5p |
|---|---|---|---|---|---|---|
| TNF-α | 0.27712 (p<0.002) | 0.2493 (p<0.01) | 0.2552 (p<0.005) | 0.2487 (p<0.005) | 0.3284 (p<0.0005) | 0.3307 (p<0.0005) |
| IL-6 | 0.2467 (p<0.01) | 0.283 (p<0.002) | 0.2568 (p<0.005) | |||
| IL-1ß | 0.2536 (p<0.005) | 0.2733 (p<0.002) | ||||
| IL-10 | 0.222 (p<0.02) | 0.2772 (p<0.002) | 0.2803 (p<0.002) |
1Circulating levels of miRNA were expressed as normalized PCR count (Ct) [a mean of PCR Ct of a miRNA measured in duplicate
subtracted of a mean PCR Ct of house-keeping gene (5S rRNA) measured in duplicate] ± standard deviation (SD).
2Correlation co-efficient by Spearman test. The significant results obtained by Spearman test were verified by Kendall tau b test.
Table 3: Association between miRNA PCR Cts and spontaneous cytokine production by monocytes in ASD subjects.
| Cytokine production | miR-193a- 5p1 | miR-379- 5p |
miR-134- 5p |
miR-382- 5p |
miR-378a- 3p | miR-423- 5p |
miR-320b |
|---|---|---|---|---|---|---|---|
| ASD (N=130) |
|||||||
| IL-6 ß-GL+LPS |
-0.23282 (p<0.001) | -0.2144 (p<0.02) | |||||
| IL-ß ß-GL+LPS |
-0.2428 (p<0.01) | -0.2242 (p<0.01) | -0.2266 (p<0.02) | -0.2305 (p<0.02) | -0.2175 (p<0.02) | -0.1852 (p<0.05) | |
| Non-ASD (N=50) |
|||||||
| IL-1ß ß-GL+LPS |
-0.2881 (p<0.05) | -0.3494 (p<0.02) | -0.3288 (p<0.05) | -0.5442 (p<0.0005) | -0.38 (p<0.01) | -0.4433 (p<0.005) | |
| IL-12 Medium only |
-0.3057 (p<0.05) | ||||||
| IL-12 zymosan |
-0.3854 (p<0.01) | -0.2877 (p<0.05) | -0.314 (p<0.05) | -0.3433 (p<0.02) | -0.4223 (p<0.005) | ||
| IL-12 ß-GL |
-0.4158 (p<0.005) | -0.3387 (p<0.02) | -0.4017 (p<0.005) | -0.4643 (p<0.002) | -0.4222 (p<0.005) | -0.4878 (p<0.0005) |
1Circulating levels of miRNA were expressed as normalized PCR count (Ct) [a mean of PCR Ct of miRNA measured in duplicate
subtracted of a mean of PCR Ct of house-keeping RNA (5S rRNA) measured in duplicate] ± standard deviation (SD).
2Correlation coefficient by Spearman test. The significant results obtained by Spearman test were verified by the Kentall tau b test. ASD
subjects did not reveal any associations between IL-12 production by monocytes and miRNA PCR Cts.
Table 4: Negative association between miRNA PCR Cts and cytokine production in non- ASD and ASD subjects.
Co-variance analysis revealed that sleep and seizures disorders were the only comorbid conditions that effected the association between miRNA PCR Cts and monocyte cytokine production (p<0.05). No other comorbid conditions did (p>0.05). Likewise, other clinical co-variables including age, gender, the use of medications (ADHD meds, anti-seizure meds, SSRIs, neuroleptics, and montelukast), and supplemental Immunoglobulin (Ig) treatment did not have an effect. The effect of supplemental Ig was tested due to the fact that some ASD subjects were diagnosed with SAD and were being all treated with supplemental Ig at the time of sample obtainment.
Effects of comorbid conditions on associations between monocyte cytokine production and circulating miRNA levels
Since co-variance analysis revealed that sleep and seizure disorders had an effect on the associations between miRNA PCR Cts and monocyte cytokine production, we further analyzed these associations in ASD subjects by grouping them as those with or without sleep/seizure disorders. We found that ASD subjects who have sleep (N=49) or seizures (N=19) disorders did not reveal any such associations, although the number of ASD subjects with seizures may be too small for such analysis. In contrast, ASD subjects who did not have either a sleep or seizure disorder revealed positive associations between monocyte cytokine production and PCR Cts of miRNAs under most culture conditions Supplementary Table 1.
This was again the most evident in cultures without stimulus (Table 5). Negative associations were detected in associations between miRNA PCR Cts and IL-6 or IL-1ß production under cultures stimulated with CLO97 or ß-glucan/LPS in ASD subjects without sleep/seizure disorders (Supplementary Table 1 and Table 5).
The effects of comorbid conditions on circulating levels of miRNAs
| ASD (N=70) | miR-193a-5p1 | miR-379-5p | miR-134-5p | miR-382-5p | miR-378a-3p | miR-423-5p | miR-320b |
|---|---|---|---|---|---|---|---|
| TNF-α | 0.46162 (p<0.0001) | 0.3968 (p<0.005) | 0.3554 (p<0.005) | 0.3419 (p<0.005) | 0.4947 (p<0.0001) | 0.4738 (p<0.0002) | 0.4256 (p<0.0005) |
| IL-6 | 0.3216 (p<0.01) | 0.3094 (p<0.02) | 0.2998 (p<0.02) | 0.2536 (p<0.05) | 0.3878 (p<0.002) | 0.3921 (p<0.002) | 0.3359 (p<0.01) |
| IL-1ß | 0.2912 (p<0.02) | 0.3019 (p<0.02) | 0.27 (p<0.05 | 0.2765 (p<0.05) | 0.3833 (p<0.002) | 0.4415 (p<0.0002) | 0.3875 (p<0.002) |
| IL-10 | 0.3943 (p<0.001) | 0.327 (p<0.01) | 0.3456 (p<0.005) | 0.3311 (p<0.01) | 0.4643 (p<0.0005) | 0.4889 (p<0.0001) | 0.4337 (p<0.0002) |
1Circulating levels of miRNA were expressed as normalized PCR count (Ct) [a mean of PCR Ct of miRNA measured in duplicate
subtracted of a mean of PCR Ct of house-keeping gene (5S rRNA) measured in duplicate] ± standard deviation (SD).
2Correlation coefficient by Spearman test. The significant results obtained by Spearman test were verified by the Kentall tau b test.
Table 5: Positive associations between miRNA PCR Cts and spontaneous production of TNF-α, IL-6, IL-1ß, and IL-10 in ASD subjects who have neither seizure nor sleep disorders.
Since the presence of sleep and seizure disorders affected the association between monocyte cytokine profiles and PCR Cts of the miRNAs measured, we further assessed if any differences existed in PCR Cts of these miRNA between ASD subjects with or without comorbid conditions (sleep disorder, GI symptoms, seizure disorder, SAD, and PANS like symptoms) (Table 6 and Supplementary Table 2). No differences were found in the PCR Cts of these miRNAs between all the ASD subjects combined and non-ASD subjects. Sixty of the ASD subjects were reported to have a seizure and/or sleep disorders while 70 ASD subjects did not. PCR Cts differed among groups of ASD subjects without sleep/ seizure disorders, those with sleep/seizure disorders, and non-ASD controls by one-way ANOVA (Table 6). This is secondary to lower PCR Cts (hence higher miRNA levels) in ASD subjects without seizure/sleep disorders (Supplementary Table 2).
| miR-193a- 5p | miR-379-5p | miR-134-5p | miR-382-5p | miR-378a-3p | miR-423-5p | miR-320b | |
|---|---|---|---|---|---|---|---|
| control (N=50) | 9.13 ± 1.23 | 12.78 ± 1.70 | 11.24 ± 1.58 | 10.48 ± 1.48 | 8.13 ± 1.27 | 5.82 ± 1.23 | 5.75 ± 1.42 |
| ASD seizure+/sleep+2 (N=60) | 9.06 ± 1.21 | 12.93 ± 1.94 | 11.22 ± 1.92 | 10.50 ± 1.83 | 8.18 ± 1.45 | 5.74 ± 1.62 | 5.77 ± 1.69 |
| ASD seizure-/sleep- (N=70) | 8.63 ± 1.11 | 12.07 ± 1.48 | 10.58 ± 1.48 | 9.80 ± 1.46 | 7.58 ± 1.48 | 5.29 ± 1.38 | 5.09 ± 1.46 |
| One-way ANOVA | p<0.02 | p<0.02 | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.02 |
1Circulating levels of miRNA were expressed as normalized PCR count (Ct) [a mean of PCR Ct of miRNA measured in duplicate
subtracted of PCR Ct of house-keeping gene (5S rRNA) measured in duplicate] ± standard deviation (SD).
2All patients diagnosed with epilepsy (seizure disorder) were confirmed to have epileptic activity by EEG.
3P values shown are obtained by Kruskall Wallis one-way ANOVA on ranks, since in most comparison, the data were not normally
distributed. The detailed results of one-way ANOVA and Krushkall Wallis one-way ANOVA on ranks were shown in Supplementary.
Table 6: Seven PCR Cts of miRNA levels selected for this study in ASD subjects with or without sleep or seizure disorders.
There were no difference in PCR Cts of these miRNAs between ASD subjects with sleep/seizure disorders and non-ASD controls.
The other comorbid conditions we examined had no effect on the circulating levels of the miRNAs in our ASD study subjects (Supplementary Table 2 and Table 6).
Significant differences observed were mainly due to lower PCR Cts in ASD subjects without seizure/sleep disorders. Comparison of this group with non-ASD controls and ASD subjects with seizure/sleep disorders by the Mann Whitney test were shown in Supplementary Table 2 along with the results of the Kolmogorov- Smirnov test.
Longitudinal changes in serum miRNA levels in ASD subjects with seizures and/or antibody deficiency
For 4 ASD subjects, we were able to obtain samples for both circulating miRNAs and monocyte cytokine profiles at 3 or 4 time points. These subjects include Case 1 (ASD with seizure disorder), Case 2 (ASD with SAD, disturbed sleep, and fluctuating PANS like behavioral symptoms), Case 3 (ASD with GI symptoms and sleep disorder), and Case 4 (ASD with GI symptoms only). In Case 1, a temporary decline of miRNA PCR Cts in parallel with a decrease in his seizure activity was reversed following viral infection. In Case 2, in parallel with the subject’s worsening behavioral and GI conditions, PCR Cts of these miRNAs remained high. PCR Cts of miRNAs declined in parallel with improving GI and sleep condition or with improvement of GI symptoms in Case 3 and Case 4, respectively. However, changes in circulating levels of these miRNAs were more evident in Case 4 who does not have sleep or seizure disorders (Figure 1).
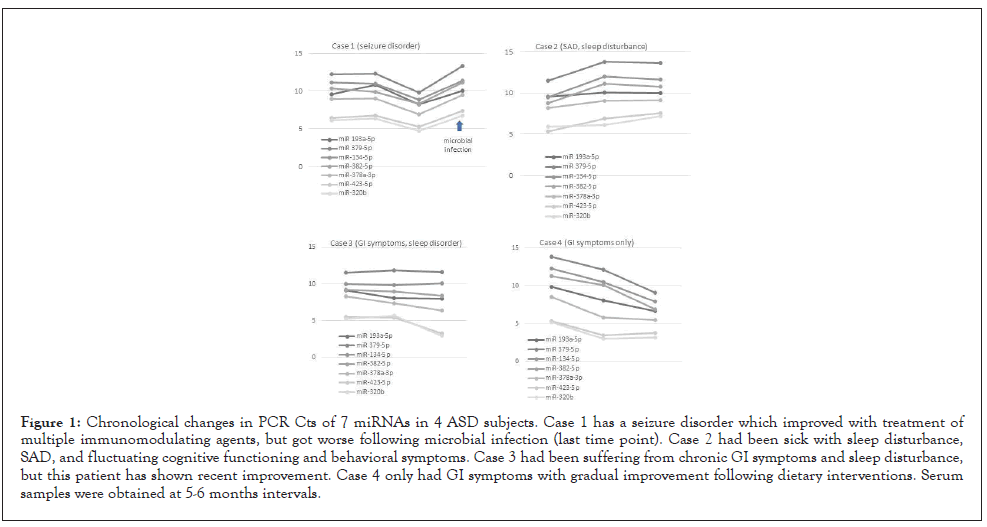
Figure 1: Chronological changes in PCR Cts of 7 miRNAs in 4 ASD subjects. Case 1 has a seizure disorder which improved with treatment of multiple immunomodulating agents, but got worse following microbial infection (last time point). Case 2 had been sick with sleep disturbance, SAD, and fluctuating cognitive functioning and behavioral symptoms. Case 3 had been suffering from chronic GI symptoms and sleep disturbance, but this patient has shown recent improvement. Case 4 only had GI symptoms with gradual improvement following dietary interventions. Serum samples were obtained at 5-6 months intervals.
This study examined how circulatory levels of 7 miRNAs, selected based on our previous results, are associated with co-morbid medical conditions and monocyte cytokine profiles [30]. Our results indicated that lower circulatory levels of these 7 miRNAs were generally associated with increased production of monocyte cytokines (TNF-α, IL-6, IL-1ß, and IL-10) in ASD subjects who do not have seizure or sleep disorders. ASD subjects with sleep or seizure disorders generally revealed low circulating levels of the miRNAs and did not reveal close correlation between monocyte cytokine profiles and circulating levels of the miRNAs studied. Our findings are summarized (Figure 2).
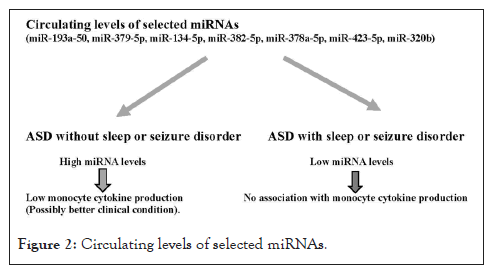
Figure 2: Circulating levels of selected miRNAs.
The effects of maternal inflammation on the fetus has been suspected in the onset and progress of ASD, as demonstrated by the MIA model, one of the well-established animal models of autism, and in human studies of maternal infection during pregnancy [31,32]. All results indicate that such effects of maternal inflammation are likely mediated by innate immunity, generated by sensing microbial byproduct and/or byproduct released by tissue injury (danger signals). Although their responses are pathogen nonspecific, potent innate immune responses can cause prolonged effects on subsequent innate immune responses via epigenetic and metabolic changes, generating IIM [33,34]. The unique nature of IIM has been implicated in neuroinflammation that is associated with common neuropsychiatric conditions [35].
IIM exerts actions on other lineage cells via secondary messengers such as cytokines. Secreted miRNAs, as a form of exosomal miRNAs, are thought to be such secondary messengers.
Circulating miRNAs are regarded as candidate biomarkers for diagnosis and monitoring disease activity in various medical conditions, secondary to their stability, something not possessed by cytokines [16]. Currently, circulating miRNAs are being used as disease markers and potential therapeutic targets in seizure disorders and in neuropsychiatric and neuroinflammatory conditions including depression/bipolar/schizophrenia, traumatic brain injury, and Rett syndrome [36-39]. It’s role as a biomarker in ASD has also been proposed [40-43]. miRNAs identified in previous studies of ASD have been shown to be associated with impaired neurogenesis and brain development. It is of note that none of the 7 miRNAs assessed in this study have been previously reported as biomarkers for ASD.
The aim of this study was to identify convenient circulatory biomarkers that can help identify the effects of comorbid conditions on neuroinflammation implicated with ASD pathogenesis. Our previous studies revealed that comorbid conditions mediated by immune responses are closely correlated to monocyte cytokine profiles. Based on our previous work, we selected 7 miRNAs which showed differences among ASD subsets categorized based on monocyte cytokine production, and were not affected by other co-variables [age, gender, ASD severity, and medications (AEDs, SSRIs, neuroleptics, ADHD medications)] [19]. In this study, we hypothesized that circulating levels of the miRNAs selected can serve as biomarkers of monocyte cytokine profiles and comorbid conditions that are closely associated with neuroinflammation.
First, we determined if any significant associations were present between the miRNA PCR Cts and monocyte cytokine production. Spontaneous production of monocyte cytokines was most notable in its association with the PCR Cts of the 7 miRNAs in the ASD subjects shown in Table 3.
Under certain culture conditions with innate immune stimuli, monocyte cytokine production also revealed significant associations with miRNA PCR Cts in the ASD subjects (Supplementary Table 1). However, further analysis revealed that positive associations between miRNA PCR Cts and production of TNF-α, IL-6, IL-1ß and IL-10) were present only in ASD subjects who did not have sleep or seizure disorders, but not in ASD subjects with sleep/seizure disorders or in non-ASD controls (Table 5 and Supplementary Table 1). These results indicate that high circulating levels of miRNAs are likely to indicate lower production of monocyte cytokines in ASD subjects without sleep/seizure disorders.
The clinical utility of the circulating miRNAs selected for this study has been mainly studied in the field of cancer research. There is limited information published regarding the roles these circulating miRNAs play in ASD, or other neuropsychiatric conditions. miR- 320b which is present in relatively high levels in circulation, has been proposed as a biomarker for both atherosclerosis and chronic obstructive lung diseases (COLD), partly reflecting its suppressive actions on cholesterol metabolism [44,45]. miR-320b in circulation has also been proposed as a biomarker of disease activity for MS [46]. Interestingly, miR-320b has been implicated in the signaling pathways of the innate immune stimuli, negatively regulating the production of IL-1ß and TNF-α in patient with COLD [47]. miR- 423-5p which is also present at a relatively high level in circulation has been implicated with the regulation of glyconeogenesis and proangiogenic activity [48,49]. High circulating levels of miR-423- 5p have also been reported in patients with sars-cov-2 infection [50]. In addition, miR-423-5p has been proposed as a potential biomarker for schizophrenia [51]. This miRNA is likely to exert regulatory actions on inflammatory conditions, partly affecting glyconeogenesis and autophagy and supports our assumption that these miRNAs serve as the regulatory 2nd messengers of IIM [52]. miR-378-3a, another miRNA that is present in relatively high levels in circulation has been implicated with suppressive actions on inflammation: in inflammatory bowel diseases, miR- 378-3a levels were reported to be decreased [53,54]. It is also of note that miR-378-3a has been reported to suppress depression like behaviors induced by chronic stress in rats [55]. miR-134-5p has been implicated in the inhibitory effects of various types of vascular inflammation, that includes rodent models of Alzheimer’s disease and dementia [56]. Finally, low circulating levels of miR-193a-5p have been shown to be associated with vascular inflammation [57,58]. Taken together, the available literature indicates that these 7 miRNAs are linked to suppression of inflammatory conditions, including neuroinflammation.
Circulating levels of these miRNAs differed among ASD subjects with or without sleep/seizure disorders and non-ASD controls and this is due to higher circulating levels of the miRNA studied in ASD subjects who did not have sleep or seizure disorders as shown in Table 6. In ASD subjects without sleep/seizure disorders, considering the suppressive actions of these miRNAs on inflammation, higher circulating levels of these miRNAs may indicate that there is better control of underlying inflammation. This assumption is supported by our findings of negative associations between circulating levels of miRNAs and monocyte cytokine production in the ASD subjects without sleep/seizure disorders. Since seizure and sleep disorders are implicated with neuroinflammation and the miRNAs studied are reported to have a role in suppressing inflammation, circulating levels of these miRNAs are expected to be low in ASD subjects with sleep/seizure disorders which is consistent with our results.
To further determine whether the miRNAs selected can be used as biomarkers of inflammation in ASD, it is necessary to assess if the changes in miRNAs PCR Cts correlate with clinical features that are associated with immune mediated inflammation. We were able to measure circulatory levels of the 7 miRNAs at 3 or 4 time points in 4 of the ASD subjects. This was due to their complex medical conditions and their repeated need for blood work. In Case 4, lowering PCR Cts paralleled with improvement of the subject’s GI symptoms (Fig. 1). In Case 1, better seizure control led to a decline of PCR Cts of these miRNAs, but PCR cts of these miRNAs increased again, following a microbial infection. In Case 2, unstable behavioral conditions along with worsening GI symptoms appear to be paralleled with persistently high PCR Cts of these miRNAs. In Case 3, improvement of severe GI and sleep disorders led to a decrease in PCR Cts of these miRNAs (Fig 1). However, such changes were not as evident as in Case 4. In patients with sleep disorders, other miRNAs that were not included in this study have been reported to play a role in circadian rhythm abnormalities [59]. Therefore, in patients with sleep disorders, measurement of additional miRNAs may be beneficial.
Nevertheless, longitudinal changes in the circulatory levels of miRNAs in these 4 ASD patients supports our hypothesis that circulatory levels of these miRNAs can serve as biomarkers of underlying inflammation. Higher circulating levels of these miRNAs may indicate compensatory suppressive mechanisms for dysregulated innate immune responses in ASD subjects, given the fact that non-ASD controls did not reveal high circulating levels of these miRNAs (Table 6).
Interestingly, others have shown that up-regulated levels of miR- 378-3p in patients with tuberous sclerosis can be normalized with the use of the mTOR inhibitor (everolimus) [60].
We are not certain why PCR Cts of these miRNAs are negatively associated with an increased production of IL-1ß, under cultures stimulated with ß-glucan plus LPS as shown in Table 6. This negative association was also observed in non-ASD controls (Table 6). In acute inflammatory conditions such as septic shock and systemic inflammatory response syndrome, circulating levels of these miRNAs increase [61,62]. Therefore, this finding may reflect normal immune responses in acute inflammation, turning on the suppressing mechanisms imposed by these miRNAs to counterregulate acute inflammatory responses.
Further research is needed to address some of the limitations of this study that includes the need for more longitudinal studies on the circulating levels of miRNAs in statistically significant numbers of ASD subjects. Additional research examining changes in the circulatory levels of these 7 miRNAs in comparison with both clinical features and monocyte cytokine profiles would also be helpful to evaluate their utility as biomarkers of neuroinflammation in ASD.
Our results revealed that the presence of seizure and sleep disorders in ASD subjects significantly affects the associations between circulating levels of 7 miRNAs selected for this study and monocyte cytokine production. High circulatory levels of these miRNAs in ASD subjects may indicate compensatory suppressive mechanisms for underlying chronic inflammation. On the other hand, persistently low circulating levels of these miRNAs may indicate ongoing uncontrolled chronic inflammation, possibly in association with sleep and seizure disorders, in ASD subjects.
The authors are thankful for Dr. L. Huguenin for critically reviewing this manuscript and Dr. G. A. Toruner for advising on the data analysis of qRT-PCR results. This research was funded by Jonty Foundation, St. Paul, MN and The Brain Foundation, Pleasanton, CA, United States (no grant number is assigned for either).
The authors declare no conflict of interest.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Jyonouchi H, Geng L (2022) Circulating MicroRNA in Autism Spectrum Disorders as a Possible Biomarker of Neuroinflammation: Association with Comorbid Conditions and Monocyte Cytokine Profiles. J Clin Cell Immunol. 13:666.
Received: 05-May-2022, Manuscript No. JCCI-22-17354; Editor assigned: 09-May-2022, Pre QC No. JCCI-22-17354 (PQ); Reviewed: 23-May-2022, QC No. JCCI-22-17354; Revised: 30-May-2022, Manuscript No. JCCI-22-17354 (R); Published: 06-Jun-2022 , DOI: 10.35248/2155-9899.22.13.666
Copyright: © 2022 Jyonouchi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.