Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Review Article - (2019)Volume 9, Issue 2
The mediastinum is the most common location of childhood intrathoracic tumors. The mediastinum is composed of three compartments: prevascular, visceral and paravertebral. Mediastinal tumors are classified according to the site of origin. At the time of diagnosis, children are often asymptomatic and lesions may be observed on screening examinations. However, large tumors can cause severe symptoms, due to local invasion and compression of vessels, nerves, trachea, bronchi and esophagus, within the mediastinum. Integration of clinical information and radiographic findings enables diagnosis. Chest radiography is obviously the first imaging procedure that is performed in children with symptoms referable to the thorax. Once a mediastinal lesion is identified by chest radiography, "cross-sectional imaging methods" are performed. In particular, computed tomography is valuable in demonstrating tumor size and morphology, its exact anatomical location and its relation with the surrounding vital mediastinal structures. This article reviews the more common childhood mediastinal tumors considering clinical manifestations and characteristic imaging findings in order to make a correct diagnosis.
Childhood mediastinal tumors; Chest X-ray; Computed tomography; Magnetic resonance imaging
Thoracic tumors in childhood are mostly located in mediastinum [1]. Mediastinal tumors in children comprise a heterogeneous group of lesions and knowledge of the exact location of the tumor within the mediastinum facilitates the diagnosis. A modern CTbased definition of mediastinal compartments has been developed by the International Thymic Malignancy Interest Group (ITMIG). The ITMIG classification system separates the mediastinum into 3 compartments: pre-vascular (anterior), visceral (intermedium) and paravertebral (posterior) [2,3]. Lymphomas and leukemias are the most common neoplasms found in the prevascular compartment, followed by Germ Cell Tumors (GCTs). Visceral compartment abnormalities include usually lymphadenopathy related to lymphoma or leukemia. Tumors of the visceral mediastinum are rarely seen in isolation and are often observed in association with anterior mediastinal disease. Finally, the paravertebral compartment is the most common location for neurogenic tumors. Childhood mediastinal tumors are localized to the paravertebral mediastinum in 52% of cases [2-6]. Mediastinum compartmentalisation help to narrow the differential diagnosis of newly detected mediastinal tumors, but it may be difficult to localize a rapidly growing tumor to its anatomic compartment because can spread from one space to another or involve the entire mediastinum [1,7]. Children are frequently asymptomatic and therefore mediastinal tumors present as incidental findings on radiographs obtained for other reasons [1,4,7]. In some cases, mediastinal tumors may present with symptoms mimicking common respiratory conditions of childhood such as cough and dispnea [1,4,8]. In children compared to adults, rapidly growing mediastinal tumors can cause easily compression of airway and blood vessels because of the smaller size of thoracic cavity. Therefore, life-threatening conditions can rapidly develop, such as Acute Respiratory Distress Syndrome (ARDS) and Superior Vena Cava (SVC) syndrome [1,5,7,9]. Other presenting symptoms can include dysphagia, if compression involves esophagus or neurological disorders due to spinal cord compression [4-7,10]. Chest X-ray is the initial imaging method when a thoracic disease is clinically suspected. The mediastinum is visualized with standard upright chest X-ray with two views: Postero Anterior (PA) and Latero Lateral (LL). Typically, only the upright PA view is performed, which enables adequate visualization of the majority of mediastinal tumors and minimizes exposure to Ionizing Radiation (IR) in pediatric patients [3,4,11-13]. Conventional standing PA view is not always possible with very young pediatric patients; in kids aged less than 3 years or with severe clinical conditions only a recumbent Antero Posterior (AP) projection may be performed [3,11]. The chest X-ray is an inexpensive, easy to perform and widely available diagnostic tool. Disadvantages include exposure to IR and low sensitivity and specificity of radiographic examination in the identification of mediastinal tumors. Chest X-ray does not provide a definitive diagnosis and necessitates additional cross-sectional imaging studies to accurately localize and characterize the lesion and narrow the differential diagnosis [3,4,6,13]. CT is the gold standard for evaluating mediastinal tumors. CT is accurately reveal the tumor size, location, and relationship with the surrounding organs and is extremely helpful to guide subsequent clinical management of young patient. The widespread use of Multi-Slides CT (MSCT) has increased the number of the exams performed to study pediatric thorax. The MSCT is a high temporal and high spatial resolution tecnique that allows faster evaluation and less sedation for kids and can be used to generate Multiplanar (MPR) and 3D reconstructions. Radiation exposure to children remains the major disadvantage of CT. Compared to adults, children are more sensitive to radiation and are highly susceptible to radiationinduced cancer since their tissues are still growing and therefore the dividing cells are more prone to somatic genetic damage. In addition, children have a longer life expectancy during which oncogenic effects may develop [3,4,11-13]. To minimize radiation dose, all radiation exposures must be justified and any necessary exposures should be kept As Low As is Reasonably Achievable (ALARA). Therefore different CT protocols are used for kids compared to adults, which reduce exposure to Ionizing Radiation with no notable impact on the quality of the exam [4,11-14]. Another important issue in radiographic evaluation of childhood mediastinal tumors is the need of sedation for kids aged less than 5 years. Sedation potentially improves the quality of images obtained and reduces the chance of repeating exam and overexposure to large amounts of IR. In children over age 5 years it is almost always possible to perform a diagnostic CT without sedation, thereby, avoiding risk of anesthesia related adverse events [4,11]. In children diagnosed with mediastinal tumor, sedation can be associated with high incidence of cardiac and respiratory complicances. CT identifies risk factors such as compression of the tumor on heart, great vessels or airways. An accurate measurement the extension of tracheobronchial narrowing predicts progressive symptoms and potential complications: on CT a decrease of more than a 50% in the cross-sectional area of the trachea correlates with respiratory symptoms such as orthopnea and stridor, intubation difficulties and altered pulmonary function [6,15]. The impaired respiratory function and/or cardiovascular complications during sedation in a pediatric cancer patient limit application of the Magnetic Resonance Imaging (MRI). Despite advantages of MRI, such as no IR exposure and high contrast resolution, the long duration of the exam and the necessity of sedation in young critically ill patients make the diagnostic technique complex and risky. Furthermore, MRI has low sensitivity in identifying calcifications and does not allow correct staging of disease because it not able to produce images of the lung that are high in spatial resolution [13,16]. MRI is primarily used to identify intraforaminal extension of neurogenic tumors, in circumstances where the use of iodinated contrast agent is contraindicated and in presence of unclear radiological findings in order to: 1) Better define relationship between tumor and heart, pericardium and large intrathoracic vessels; 2) Differentiate between cystic and solid lesions; 3) Identify cystic/necrotic components within solid tumors [1,3-5,13,16,17].
Lymphomas and leukemias
Lymphomas represent 13% of all pediatric cancers and most commonly occur in the prevascular mediastinum. They can be divided in Hodgkin Disease (HD) and Non-Hodgkin Lymphoma (NHL). HD is more common in adolescents, while NHL occurs more frequently in childhood [1,2-7,12,13,18]. Lymphomas are systemic diseases and clinical presentation depends on regions of disease involvement. The mediastinum is involved in 85% of all cases of HD and 50% of all cases of NHL. Nodular sclerosis is the most frequently diagnosed subtype of HD, while T-cell lymphoblastic lymphoma (T-LL) is the subtype of NHL that commonly affects the mediastinum [3,6,7,12,18-20]. HD usually has a slow clinical course and in the majority of cases it presents cervical and/or supraclavicular lymphadenopathy; B symptoms, such as fever, night sweats and/or weight loss are present in 25%-30% of patients. At diagnosis, mediastinal involvement, if present, is clinically silent since compression of bronchial and vascular structures occurs slowly [12,19-22]. In contrast, NHL is typically an aggressive and rapidly growing disease. 70% of kids with T-LL present with a rapidly growing tumor in the prevascular mediastinum: patients frequently have symptoms due to compression of the mediastinal bronchial and/or vascular structures such as persistent dry cough and dyspnea and the disease may be complicated by ARDS or SVC syndrome. In addition, pleural and/or pericardial effusions are frequent as well as systemic B symptoms such as fever, malaise, fatigue, weight loss [6,9,19,20,22]. T-cell Lymphoblastic Leukemia (T-ALL) can show clinical similarities with T-LL. Children with T-ALL can present with the signs or symptoms referable to tracheobronchial compression or SVC obstruction due to anterior mediastinal mass; pleural and pericardial effusions are often seen. The distinction between the two diseases is arbitrary and based on the extent of involvement of Bone Marrow (BM): patients with less than 25% blasts in BM are diagnosed as NHL [6,19,20,23]. Both chest X-ray and CT are required for evaluation and diagnosis of mediastinal lymphoma/leukemia. Chest X-ray provides preliminary information about mediastinal involvement. Frontal chest radiograph can show widening of the monolateral or bilateral mediastinum it typically manifests as a lobulated soft tissue mass or as area of increased opacity with net or blurred margins; furthermore, a large anterior mediastinal mass may result in compression and deviation of the trachea and the loss of normal borders of the anterior mediastinum, that is, the ascending aorta, right and left heart border. Lateral chest radiograph, if it is performed, can demonstrate the anterior location of the tumor, based on obscuration of the retrosternal clear space [3,4,7,12,13,18,21]. In the past, a mediastinal mass was defined as “bulk” if the tumor was larger than one-third of the thoracic diameter measured transversely at the dome of the diaphragm on upright PA chest X-ray [19,20]. Currently, according to the EuroNet- PHL-C2 protocol for pediatric and adolescent HD, “bulk” is referred to the volume of the largest contiguous lymph node mass measured using CT imaging [12]. In children, a normal thymus can vary greatly in size and symmetry and is sometimes mistaken for true pathology causing superior anterior mediastinal widening [3,5,12,13,18-20]. An enlarged thymic shadow, which was not previously seen on radiogram, is highly suspicious for malignancy [7]. The normal thymus is typically visualized on chest X-ray until the age of 5, when lymphomas are rare. On chest X-ray the thymus appears as a homogeneous soft tissue density, merging with the heart’s projection without compression of adjacent structures. In the majority of cases, an accurate analysis of medical history, the revision of previous radiographs and an eventual ultrasound can clarify any diagnostic doubt [3,4,7,12,13,18-20,24]. In addition, chest X-ray enables evaluation of pulmonary parenchymal involvement, which is present at the time of diagnosis, in 5-10% of patients with HD and in 5% of patients with NHL. Multiple nodules with irregular borders are the most common finding on chest radiograph. An increased reticular interstitial thickening is rarely present and can also infrequently occur a parenchymal consolidation, which can resemble pneumonia [4,12,18-20]. Pleural effusion typically arises from venous or lymphatic obstruction caused by hilar or mediastinal adenopathy; at the time of diagnosis pleural effusion is a rare finding in patients with HD, whereas it occurs frequently in children with T-LL or T-ALL, sometimes associated to pericardial effusion. Therefore, in children with suspected T-LL or T-ALL, chest X-ray must be performed emergently [6,12,18-20,22]. Ultimately, radiographic findings can be nonspecific: mediastinal enlargement can be the result of other malignant or benign conditions and differential diagnosis is really not possible. Moreover, chest X-ray may lead to misdiagnosis, such as in cases of: 1) Small volume tumors non visible or obscured by pleural and/or pericardial effusions; 2) Small pulmonary and/or pleural lesions not apparent; 3) Small amounts of pleural effusion not detected; 4) Lymphomatous infiltration of thoracic wall, even if quite rare, with bone erosions or soft-tissue lesions no appreciable [6,12]. CT with intravenous (IV) contrast is without doubt the imaging modality of choice in the evaluation and characterization of anterior mediastinal tumors: it typically shows the lymphoma and leukemia as large, smooth or lobulated, soft tissue tumors, representing thymic and lymph node enlargement. In addition, CT can show heterogeneous enhancement with low attenuation areas within the tumor, representing haemorrhage, necrosis or cystic degeneration; calcifications can be observed after treatment [3- 7,12,13,18-20]. According to EuroNet-PHL-C2 protocol, bulk is present if the volume of the largest contiguous lymph node mass is ≥ 200 mL. Volumes are measured using the three largest perpendicular diameters on multiplanar reconstruction mode in CT/MRI (Bulk volume calculation=AxBxC/2 ≥ 200 mL) (Figures 1 and 2) [12]. CT is valuable in detecting compression of vital mediastinal structures by mediastinal tumor, particularly in children affected by T-LL or T-ALL (Figures 3 and 4). Finally, CT accurately evaluates the presence of small parenchymal nodules, pleural or pericardial effusion, chest wall involvement and pleural and pericardial infiltration [3- 7,9,15,18-20,23]. Thymic hyperplasia, which can occur as a rebound effect after chemotherapy and/or radiotherapy must be considered in the differential diagnosis of recurrence of mediastinal lymphoma/ leukemia. Chih-Ho Chen et al. reported that Rebound Thymic Hyperplasia (RTH) developed in 67.7% of pediatric patients with lymphoma in Complete Remission (CR) after chemotherapy. In their study, interval from the cessation of chemotherapy to the identification of RTH ranged from 1 month to 17 months and the thymic hyperplasia persisted from 3 months to 5 years and gradually faded [12,25]. On CT, a solid formation in the anterior mediastinum, which preserves bilobed configuration and has the same density as muscle, is considered indicative of thymic hyperplasia. In addition, the homogenous soft tissue attenuation of the thymus seen on CT of children becomes heterogeneous as fatty infiltration begins after puberty. Occasionally the enlarged thymus can compress and displace the adjacent structures. Therefore, rebound thymic enlargement may be difficult to discern from lymphoma/leukemia recurrence [3,5,7,12,13,19,20,25]. MRI is useful to visualized the thymus when it contains fat, because thymic hyperplasia shows loss of signal on out-of-phase sequences caused by suppression of microscopic fat interspersed between non neoplastic thymic tissue, while lymphoma and leukemia do not suppress on out-of-phase sequences [3,7,16,17,24,25]. In the majority of cases of children with prior lymphoma or leukemia, a diagnosis of “Rebound Thymic Hyperplasia” is supported in situations where the clinical presentation does not suggest relapse, together with characteristics of shape and density of thymic hyperplasia and a progressive reduction in the size of the thymus at follow up CT; few such cases require biopsy [12,18-20,25].
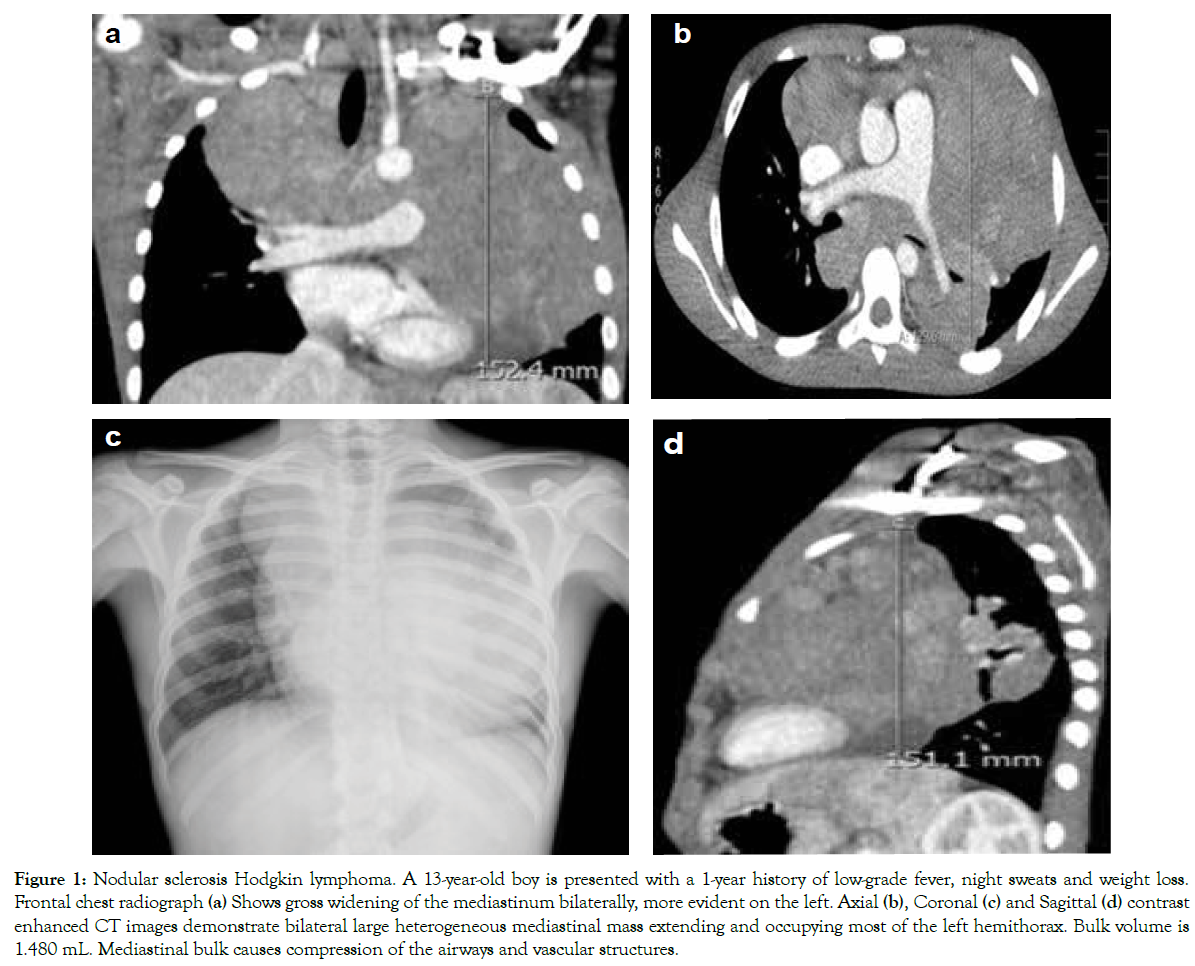
Figure 1: Nodular sclerosis Hodgkin lymphoma. A 13-year-old boy is presented with a 1-year history of low-grade fever, night sweats and weight loss. Frontal chest radiograph (a) Shows gross widening of the mediastinum bilaterally, more evident on the left. Axial (b), Coronal (c) and Sagittal (d) contrast enhanced CT images demonstrate bilateral large heterogeneous mediastinal mass extending and occupying most of the left hemithorax. Bulk volume is 1.480 mL. Mediastinal bulk causes compression of the airways and vascular structures.
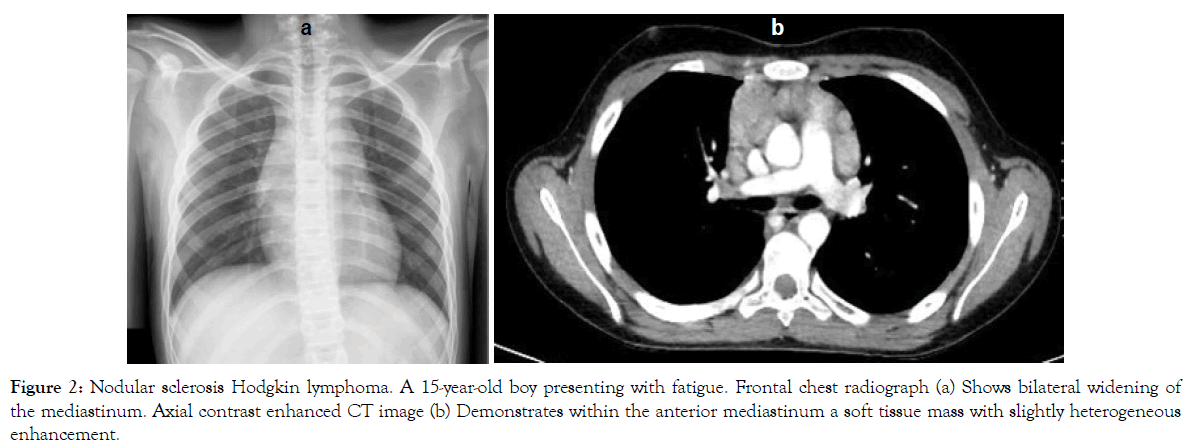
Figure 2: Nodular sclerosis Hodgkin lymphoma. A 15-year-old boy presenting with fatigue. Frontal chest radiograph (a) Shows bilateral widening of the mediastinum. Axial contrast enhanced CT image (b) Demonstrates within the anterior mediastinum a soft tissue mass with slightly heterogeneous enhancement.
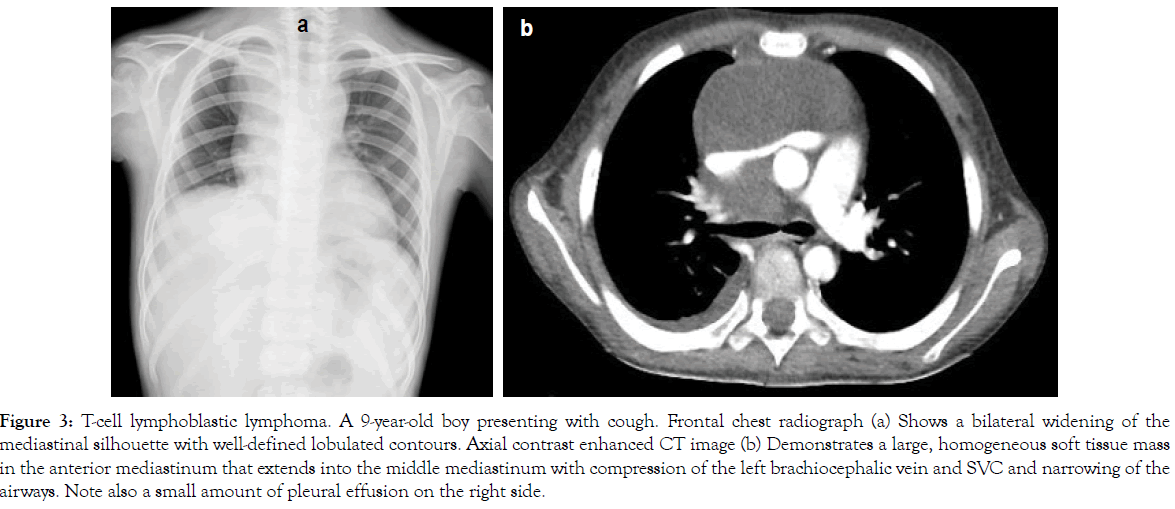
Figure 3: T-cell lymphoblastic lymphoma. A 9-year-old boy presenting with cough. Frontal chest radiograph (a) Shows a bilateral widening of the mediastinal silhouette with well-defined lobulated contours. Axial contrast enhanced CT image (b) Demonstrates a large, homogeneous soft tissue mass in the anterior mediastinum that extends into the middle mediastinum with compression of the left brachiocephalic vein and SVC and narrowing of the airways. Note also a small amount of pleural effusion on the right side.
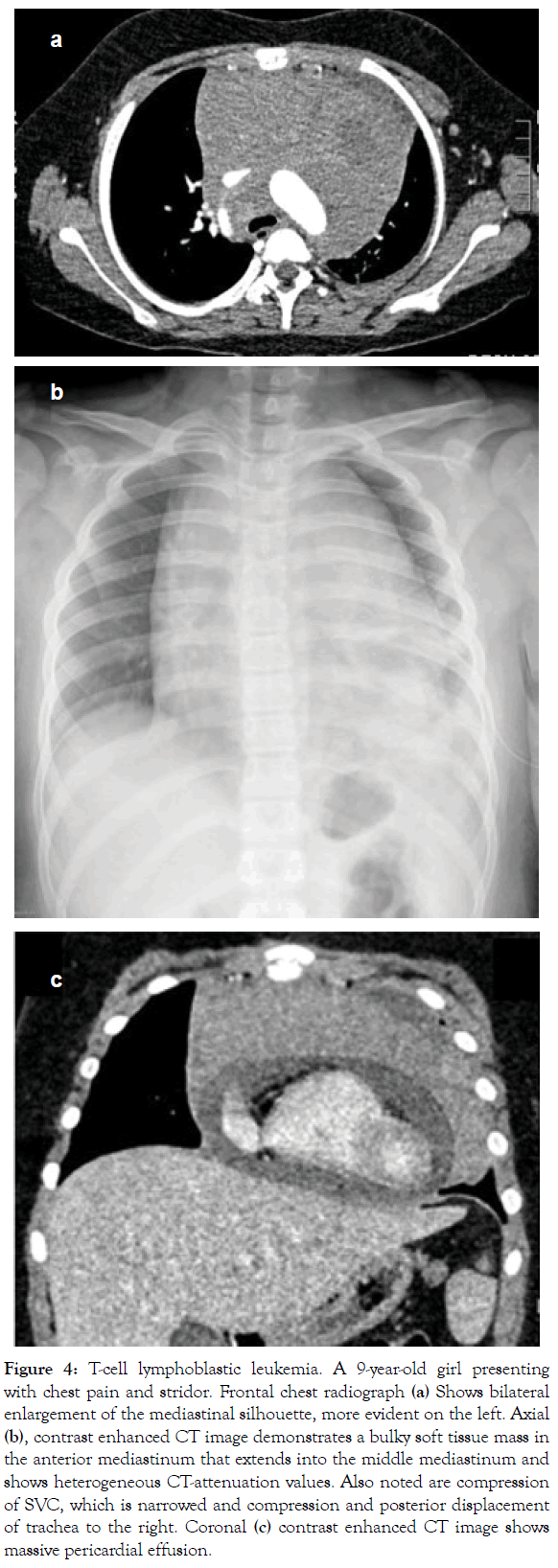
Figure 4: T-cell lymphoblastic leukemia. A 9-year-old girl presenting with chest pain and stridor. Frontal chest radiograph (a) Shows bilateral enlargement of the mediastinal silhouette, more evident on the left. Axial (b), contrast enhanced CT image demonstrates a bulky soft tissue mass in the anterior mediastinum that extends into the middle mediastinum and shows heterogeneous CT-attenuation values. Also noted are compression of SVC, which is narrowed and compression and posterior displacement of trachea to the right. Coronal (c) contrast enhanced CT image shows massive pericardial effusion.
GCTs represent 3% of neoplasms diagnosed in children and adolescents less than 15 years old and mediastinal primary GCTs account for 1%-3% of all GCTs [6,7,26,27]. Furthermore, GCTs represent 6%-18% of all mediastinal tumors and are the second most common cause of prevascular lesions [1,3,6,7,13,18,28]. GCTs are typically located within or near the thymus gland in the anterior mediastinum and can be divided into four groups: 1) Teratomas; 2) Seminomas; 3) Non-seminomatous tumors, which are represented by Endodermal Sinus Tumor (EST), embryonal carcinoma and choriocarcinoma; 4) Mixed tumors, where different components are present in the same tumor [3,5-7,13,18]. Teratomas are the most common mediastinal Germ Cell Tumors and account for approximately 25% of anterior mediastinal masses in children [4,5,7]. Teratoma can be composed of cells derived from all three germinal layers (ectoderm, mesoderm and endoderm), but most commonly, elements from only two layers are found. Teratomas are classified as: 1) Mature, if tissues are well-differentiated; 2) Immature, if tissues are not completely differentiated, with the typical presence of neuroectodermal tissue and blastema; 3) Teratoma with malignant transformation [4,5,7,27,28]. The mature type is most common, representing 45%-80% of mediastinal GCTs [3,18,29]. Teratomas can contain hair, skin, neural tissue, bone, cartilage, teeth, muscle, fat, bronchial and intestinal epithelium, thymic and endocrine tissue, in variable proportions; endocrine and exocrine pancreatic tissue is sometimes observed in mediastinal teratomas [4,5,27,30-34]. Mature and immature teratomas, even if histologically benign and with normal Alpha Feto Protein (αFP) and Beta Chorionic Gonadotropin (βHCG) blood levels, have high risk of local relapse when surgical resection is incomplete (mostly for immature histotype). Relapses can contain benign teratoma tissue and can also have a malignant component represented by: 1) Malignant elements of GCT (EST, embryonal carcinoma, choriocarcinoma, seminoma or variable combination of these entities); 2) Malignant somatic transformation (carcinomas, sarcomas, embryonal tumors). Elevated αFP occurring in relapses indicates the presence of microscopic foci of EST even without histological confirmation [29]. The age at onset is between 1 and 73 years; average age at diagnosis is 28 years; most mediastinal teratomas identified in utero or within the first year of life are immature. Mature teratoma has the same frequency in both sexes, while immature teratoma almost exclusively affects males [4,27,31]. Respiratory symptoms are almost always present in newborns, while children more than 2 years old are often asymptomatic; only patients with a large mediastinal mass present cough, dyspnea and/ or chest pain at diagnosis and teratomas rarely cause SVC syndrome [4,18,27,28,30,31]. Trichoptysis (cough productive of hair and/or sebaceous material) is rare but pathognomonic for a teratoma; it may be present after developing communication between the tumor and the tracheobronchial tree due to the secretion of digestive enzymes released from pancreatic tissue within the teratoma [27,30,31,35-37]. If the enzyme-induced erosions cause tumor rupture into the tracheobronchial tree, pleural cavity, pericardium, and lung parenchyma, may develop recurrent tracheobronchitis, pleural effusion, pleuritis, empyema, pericarditis, pericardial effusion and pneumonia. Bacterial infection complicating mediastinal teratoma rupture can also occur [3,4,27,37]. Some teratomas can cause hypoglycemia or precocious puberty due to secretion of insulin and βHCG, respectively. Increased βHCG and precocious puberty are more frequent in non-seminomatous tumors and can be sometimes observed in patients with Klinefelter syndrome [6-7,28,33,34,36,38]. Approximately 20% of mediastinal GCTs are malignant (MGCTs) and consist of seminomas and nonseminomatous tumors. They have a bimodal age distribution with a peak during infancy and a peak after puberty. Most MGCTs occur in adolescent boys. Non-seminomatous Germ Cell Tumors are largely predominant as compared to seminomatous tumors, rarely seen before puberty [5,6,26]. MGCT can be very large and cause symptoms due to compression or invasion of adjacent mediastinal structures. Most common symptoms at diagnosis are: dyspnea (25%), chest pain (23%), cough (17%), fever (13%), weight loss (11%), SVC syndrome (6%) and weakness or fatigue (6%). Less common symptoms are: thoracic wall or cervical swelling (2%), hemoptysis, hoarseness or dysphagia (1%) [1,5-7,18,36]. Non-seminomatous tumors are often associated with increased serum βHCG or αFP levels, while seminomas present with small elevation of the marker βHCG; therefore, high αFP level suggests the presence of non-seminomatous MGCT and determination of the markers can facilitate the diagnosis [3,5,6,36]. Chest X-ray is the initial diagnostic modality for the evaluation of mediastinal GCTs. On radiographic image, mature and immature teratomas appear as rounded or lobulated, well circumscribed tumors and can sometimes be large in size; dystrophic calcifications are seen in 26% of cases and may be helpful in suggesting the correct diagnosis [3,4,7,18]. On chest X-ray, MGCT appear as a mass with lobulated borders and calcifications are rare; it is frequently very large and sometimes extended to both sides of the midline [5-7,36,39]. CT with IV contrast allows better evaluation of GCTs. It is able to: 1) Distinguish different tissue components; 2) Evaluate extension of the disease; 3) Identify complications [18]. On CT, mature teratoma is seen as sharply marginated, spherical, and lobulated anterior mediastinal tumor and frequently appears as a cystic lesion with a variable wall thickness and calcifications within the wall (Figure 5). Immature teratoma usually appear as multilobulated solid tumor. In addition, immature and mature teratomas can cause mass effect on surrounding structures and can be heterogeneous: the presence of an anterior mediastinal mass composed of fluid, varying degrees of soft tissue, fluid/cystic densities, calcifications and fat is strongly suggestive for the diagnosis of teratoma [3,4,7,27,31,36,37] (Figure 6). On CT, seminomas often appear as large, lobulated anterior mediastinal tumors that rarely calcify and typically exhibit homogeneous contrast enhancement; in addition, usually do not invade adjacent mediastinal structures [3,5-7,36,39]. At CT imaging, non-seminomatous MGCTs present as bulky, irregular masses with extensive heterogeneous areas of low attenuation caused by necrosis, hemorrhage, and cyst formation; they are very aggressive tumors, frequently cause a marked mass effect and infiltrate into surrounding vital mediastinal structures such as the heart and great vessels, trachea and main bronchi, venous and lymphatic structures [3,5-7,28]. The diagnostic evaluation should integrate clinical, laboratory, and imaging data. CT alone cannot distinguish between seminoma or non-seminomatous tumors and other lesions involving the anterior mediastinum and tumor markers determination can help identifying non-seminomatous tumors. In addition, it is difficult to distinguish between primary or secondary mediastinal lymphoma or primitive mediastinal seminoma and metastatic involvement of mediastinal lymph nodes of gonadal GCT, although it is a very rare condition [3,5,6,36,39].
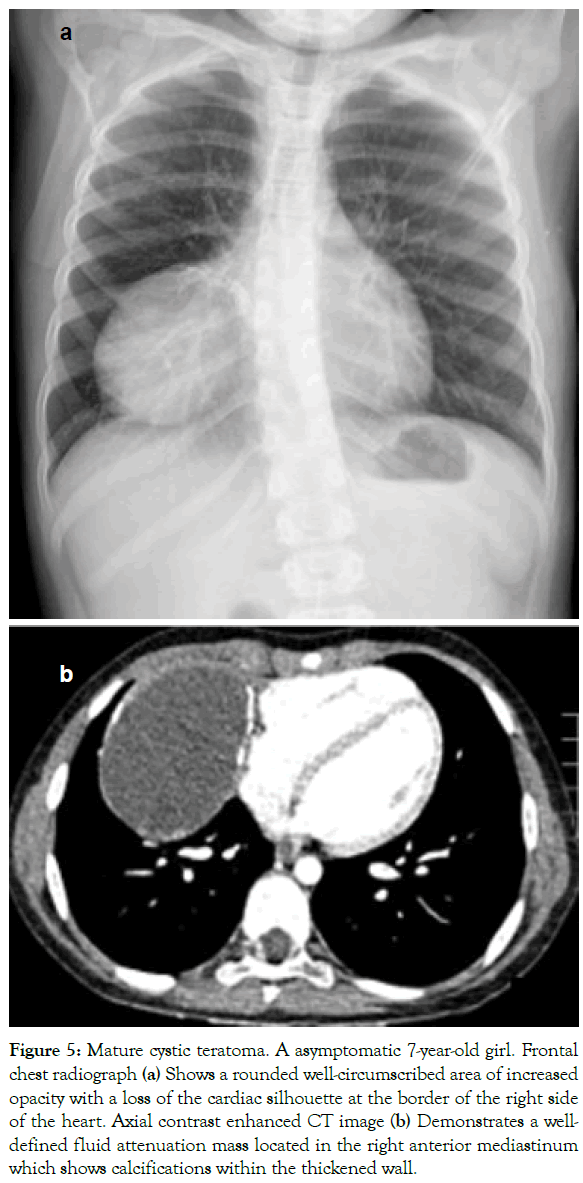
Figure 5: Mature cystic teratoma. A asymptomatic 7-year-old girl. Frontal chest radiograph (a) Shows a rounded well-circumscribed area of increased opacity with a loss of the cardiac silhouette at the border of the right side of the heart. Axial contrast enhanced CT image (b) Demonstrates a welldefined fluid attenuation mass located in the right anterior mediastinum which shows calcifications within the thickened wall.
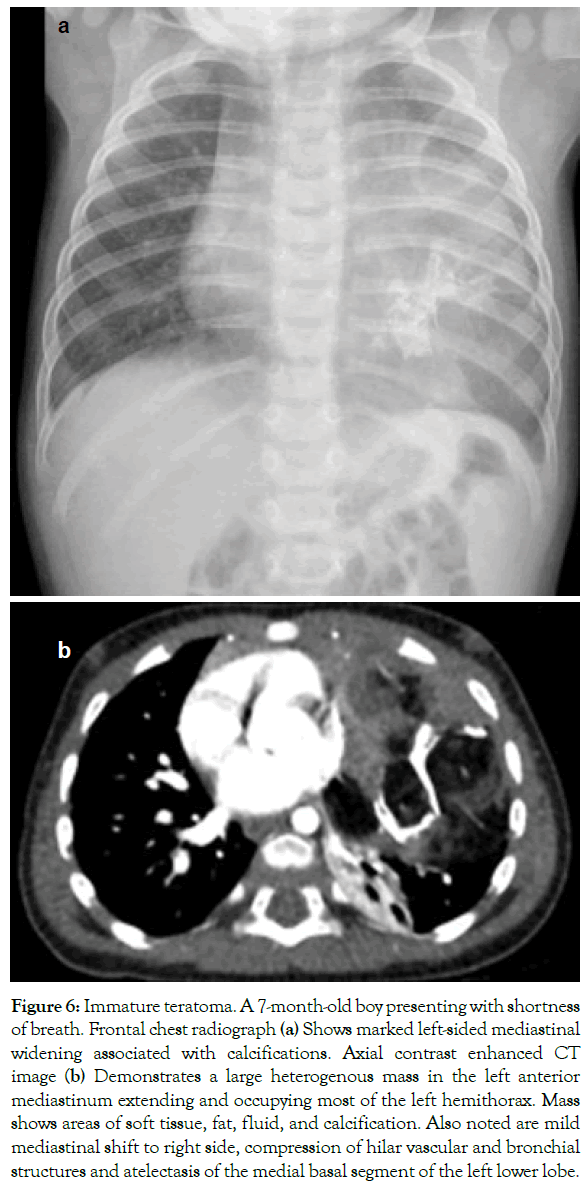
Figure 6: Immature teratoma. A 7-month-old boy presenting with shortness of breath. Frontal chest radiograph (a) Shows marked left-sided mediastinal widening associated with calcifications. Axial contrast enhanced CT image (b) Demonstrates a large heterogenous mass in the left anterior mediastinum extending and occupying most of the left hemithorax. Mass shows areas of soft tissue, fat, fluid, and calcification. Also noted are mild mediastinal shift to right side, compression of hilar vascular and bronchial structures and atelectasis of the medial basal segment of the left lower lobe.
Neurogenic tumors represent about 90% of all posterior mediastinal masses in childhood; they derive predominantly from the paravertebral sympathetic chain ganglia and are classified into Neuro Blastoma (NB), ganglio Neuro Blastoma and ganglioneuroma based on the grade of tumor tissue differentiation. In children, nerve sheath tumors, such as schwannomas and neurofibromas, are rare [1,3-7,10,13,16,18,40,41]. NB is the most common neurogenic tumor in infants and children, representing about 10% of all pediatric cancers; it arises predominantly in the abdomen and can occur in the chest in 20% of cases [1,3-7,10,13,40- 42]. Incidence is highly age dependent, peaking during infancy and rapidly declining with older age. Almost 90% of Neuro Blastomas (NBs) are diagnosed before the age of 5 and median age at onset of the disease varies between 1 and 2 years. NB is very common in children less than one year of age and can also be found before birth during a routine ultrasound; in addition, nearly 50% of the tumors diagnosed in children younger than two years can spontaneously regress. Males have a slightly higher incidence of NB than females [1,4-7,10,40-42]. Patients are often asymptomatic and tumor is discovered incidentally on routine chest radiograph. When symptoms occur, they are related to the prolonged compression by posterior mediastinal mass and are characterized by cough, dyspnea, chest pain and dysphagia; if stellate ganglion is involved, Horner's syndrome can be present (ptosis, miosis, anhidrosis). In 10%-15% of the cases NBs can extend into the neural foramina and can compress nerve roots and the spinal cord leading to neurological symptoms as: muscular hypotonia, hyporeflexia or areflexia, spasticity, dorsal radicular pain and progressive paralysis [4-7,10,40-42]. At the time of diagnosis, approximately 50% of patients present widely metastatic disease. NB metastasizes by both lymphatic and hematogenous routes and most common metastatic sites include the regional nodes, bone, Bone Marrow and liver; orbital metastatic Neuro Blastoma may cause unilateral or bilateral proptosis and periorbital or eyelid ecchymosis ("raccoon eyes"). In addition, in children, NB is the most frequent malignancy to cause skin metastases and metastatic spread to the skin manifests as blue subcutaneous nodules, known as “blueberry muffins”, which are rare in older patients [5,10,42,43]. Children with disseminated Neuro Blastoma may have systemic manifestations, including malaise, fever, weight loss, pallor and bone pain. NB may secrete catecholamines at low levels that rarely are sufficient to cause symptoms as hypertension, tachycardia, sweating, rash and irritability. However, most children excretes large amounts of catecholamine metabolites such as Vanillyl Mandelic Acid (VMA) and Homo Vanillic Acid (HVA) into urine. The measurement of urinary levels of these metabolites is widely used for the diagnosis and monitoring of NB in children. Finally, although rarely, NB may present with paraneoplastic syndromes. Opsoclonus-myoclonus is the most common neurological paraneoplastic syndrome, which occurs when antibodies cross-react with cerebellar tissue; syndrome is characterized by rapid involuntary eye movements in all directions (opsoclonus) and jerky movements of the trunk and limbs (myoclonus). Most children with opsoclonus-myoclonus have less aggressive NBs and good survival rates, but suffer from long-term neurologic sequelae. Another paraneoplastic syndrome seen with NB is intractable diarrhea with electrolyte disturbances due to release of Vasoactive Intestinal Peptide (VIP) [4-7,41,42]. Conventional radiograph can provide key information for diagnosing NB. NB is visible on frontal radiograph of the chest as a well circumscribed, spherical or lobulated, paraspinal opacity. Calcifications are visible in 30% of cases and there are frequently rib and vertebral erosions and splaying of the adjacent posterior ribs. Lateral chest radiograph can show that the paraspinal opacity is overlapping lower thoracic vertebrae suggesting posterior mediastinal location of the tumor. The intervertebral foramen may appear enlarged on the lateral view, due to intraspinal extension of the NB [3,4,6,7,10,13,18,40-42]. Cross-sectional imaging with CT confirms the presence of the tumor and evaluates extent of disease. On CT, Neuro Blastoma appears as well-defined, lenticularshaped soft tissue tumor in the posterior compartment of the mediastinum; after the IV contrast agent injection, the tumor can show heterogeneous enhancement with low attenuation areas, representing haemorrhage and/or necrosis. With CT, calcifications can be seen in up to 80% of patients. Additionally, extension of a NB through the adjacent intervertebral foramen into the spinal canal can occur in a "dumbbell" configuration, which is seen on CT and it occurs in up to 28% of patients with thoracic Neuro Blastoma [3-5,7,10,13,18,40,42]. MRI enables better visualization of NB extension into the neural foramen and spinal canal and provides accurate evaluation of marrow involvement. On MRI, NB is typically heterogeneous in signal with a low signal on T1 and a high signal on T2 weighted sequences. In addition, NB exhibits variable enhancement after contrast material administration (Figures 7 and 8). Children with suspected spinal canal involvement require urgent MRI because early surgical treatment prevents neurological long-term sequelae; however, MRI has a lower sensitivity than CT in depicting bone erosions [3-5,7,10,13,16-18,41,42].
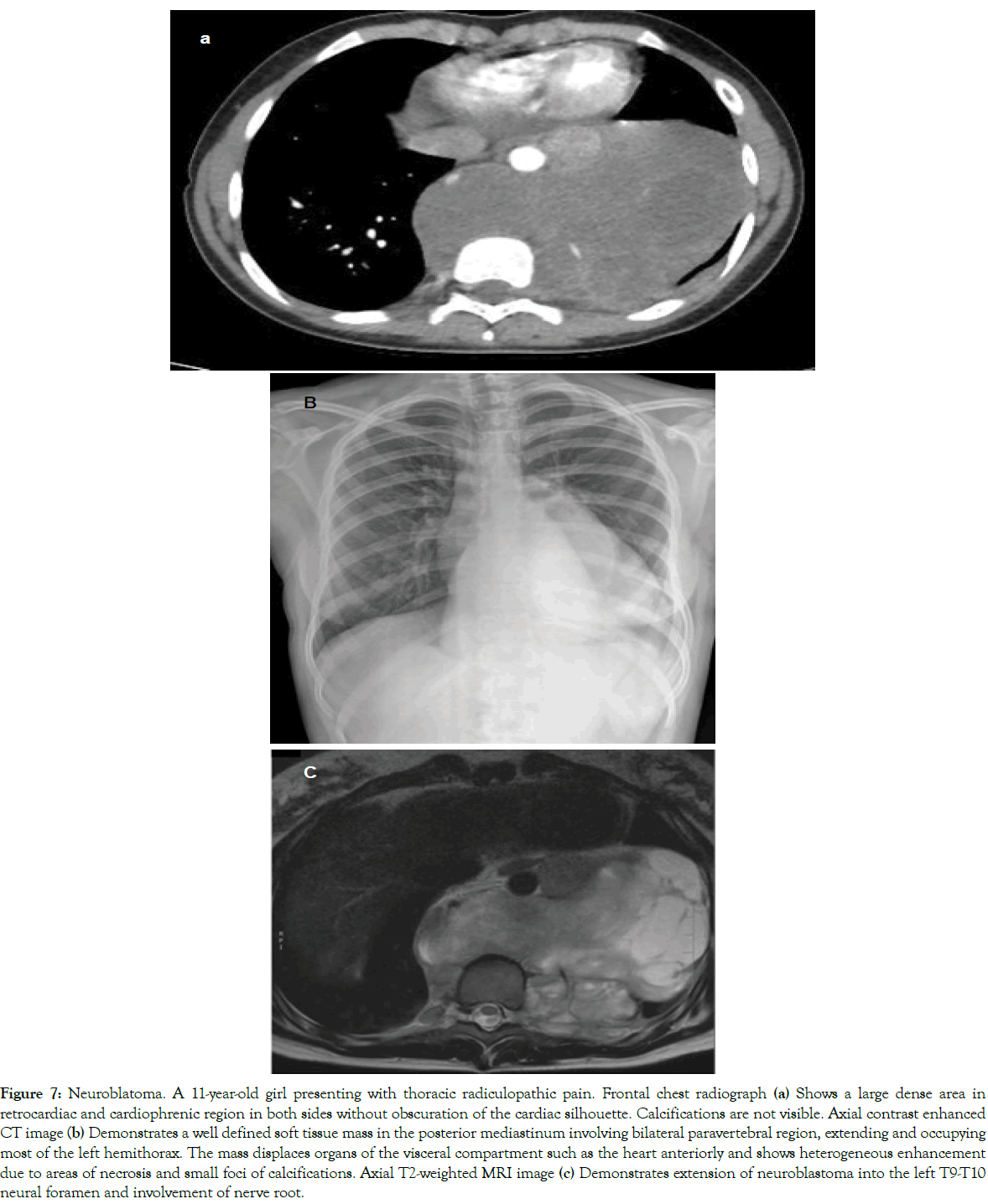
Figure 7: Neuroblatoma. A 11-year-old girl presenting with thoracic radiculopathic pain. Frontal chest radiograph (a) Shows a large dense area in retrocardiac and cardiophrenic region in both sides without obscuration of the cardiac silhouette. Calcifications are not visible. Axial contrast enhanced CT image (b) Demonstrates a well defined soft tissue mass in the posterior mediastinum involving bilateral paravertebral region, extending and occupying most of the left hemithorax. The mass displaces organs of the visceral compartment such as the heart anteriorly and shows heterogeneous enhancement due to areas of necrosis and small foci of calcifications. Axial T2-weighted MRI image (c) Demonstrates extension of neuroblastoma into the left T9-T10 neural foramen and involvement of nerve root.
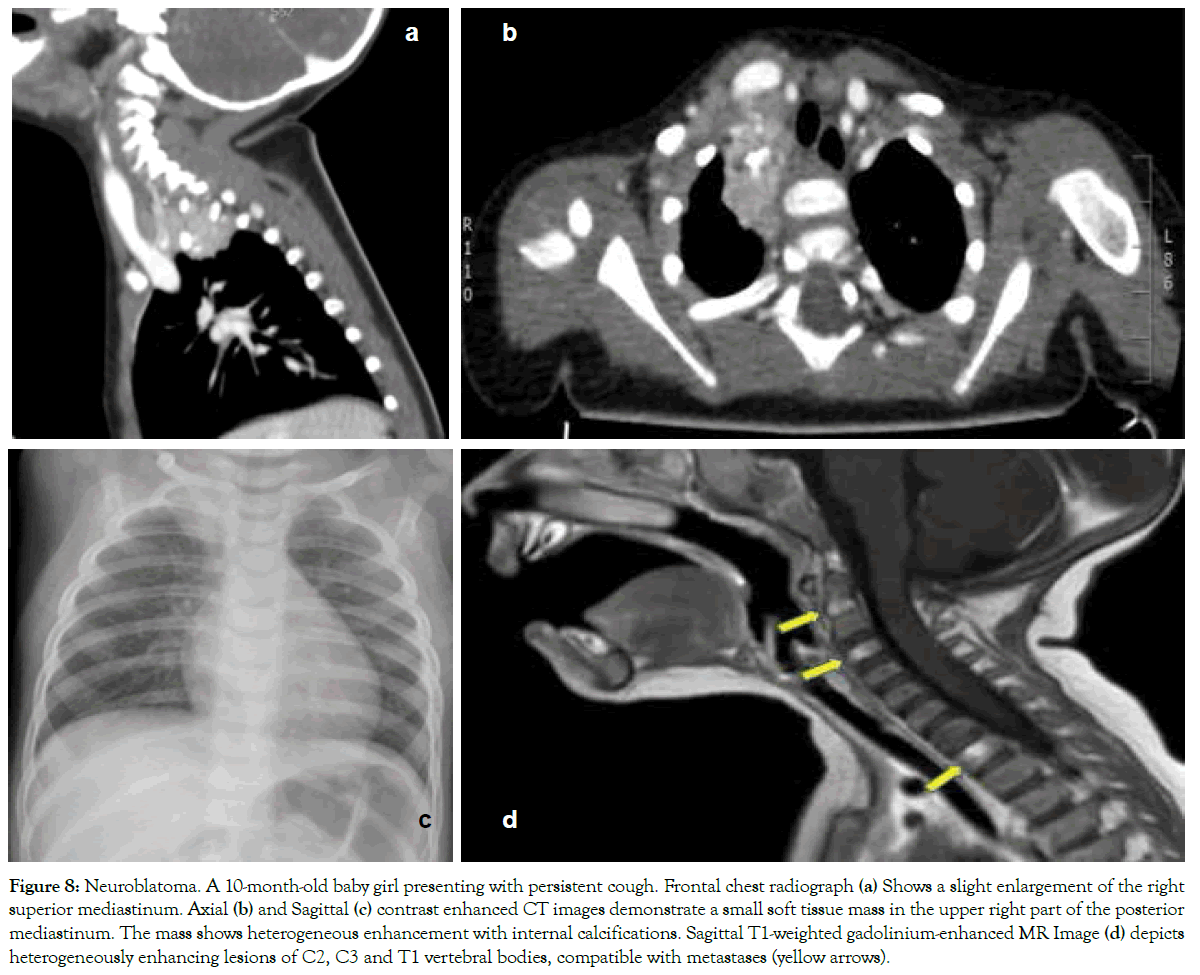
Figure 8: Neuroblatoma. A 10-month-old baby girl presenting with persistent cough. Frontal chest radiograph (a) Shows a slight enlargement of the right superior mediastinum. Axial (b) and Sagittal (c) contrast enhanced CT images demonstrate a small soft tissue mass in the upper right part of the posterior mediastinum. The mass shows heterogeneous enhancement with internal calcifications. Sagittal T1-weighted gadolinium-enhanced MR Image (d) depicts heterogeneously enhancing lesions of C2, C3 and T1 vertebral bodies, compatible with metastases (yellow arrows).
Most common childhood mediastinal tumors arise from neurogenic, lymphatic and germinal tissues. The clinical presentation ranges from a complete lack of symptoms to severe life-threatening conditions. Imaging plays a vital role in the identification and characterization of childhood mediastinal tumors, which can often be diagnosed based on location and radiological features alone. Additionally, in symptomatic children imaging is essential in making a rapid diagnosis in order to plan treatment and to avoid the severe complications of the disease.
Conflict of interest the authors declare that they have no conflict of interest.
Author is grateful to families who participated in the study.
This article does not contain any studies with human or animal subjects performed by any of the authors. Informed consent Additional informed consent was obtained from all the patients for which identifying information is not included in this article.
Citation: Brillantino C, Rossi E, Tambaro FP, Minelli R, Bignardi E, Cremone G, et al. (2019) Clinical and Imaging Findings Useful in the Differential Diagnosis of Most Common Childhood Mediastinal Tumors. Trans Med 9:207.
Received: 20-May-2019 Accepted: 04-Jun-2019 Published: 12-Jun-2019
Copyright: © 2019 Brillantino C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.