Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2021)Volume 6, Issue 11
Introduction: Urinary Tract Infection (UTI) in neonates (<30 days of age) is related to bacteremia and congenital malformations of the kidneys and the urinary system. Upper urinary tract infections cause pyelonephritis, which may lead to scaring of the renal parenchyma and eventually to chronic renal disease. In neonates that were born, the prevalence of UTI ranges from 7% to 15% in different studies. The risk of UTI is higher among prematures and low birth weight neonates. In this study we examine closely the microbiological properties and symptoms of primary infection in young infants with predisposition to recurrent infection.
Methods: Our study is a retrospective study that included infants with urinary tract infection in the first year of life, 222 infants were hospitalized during 2011-2016.
Results: 222 infants were enrolled; (35%) of the infants treated were <2 months old. Infants with urinary tract infection (20%) recurrent infections were seen. Laboratory findings have shown that the E-coli bacterium is the main contaminant. And further Klebsiella, Enterococcus, Proteus mirabilis. Note that in terms of susceptibility Klebsiella and E-coli bacteria showed high sensitivity to cephalospirins, resprim and 70% to tazobactam. Resistant bacteria such as Klebsiella and ESBL are shown to be susceptible to tzobactam, and aminoglycosides especially amikacin and garamycin in 20%-30% especially in infants with recurrent urinary tract infections in the first year. Noting the common contaminating bacteria are E-coli and Klebsiella, and in the recurrent infections these bacteria were observed more resistance especially to aminoglycosides and Tazocin.
UTI; Premature; Pyelonephritis; Antibiotics; Infants
The most common bacterial cause of community acquired UTI in term neonates is E.coli, which is responsible for 80% of the infections. Other gram-negative bacteria, such as Citrobacter, Klebsiella, Proteus and Enterobacter are also associated with UTI [1,2]. A hematogenous spread of infection is considered to be a cause of UTI, due to a higher incidence of febrile infections, which are related to bacteremia. However, there is another hypothesis, in which the cause of UTI is an ascending infection and not hematogenous spreading. This hypothesis is relying on the microbiology of these infections and the higher incidence of accompanied urinary tract malformations. The hematogenous spread has a main role in causing UTI among prematures, compared to neonates. About 75% of all neonates affected by UTI aged 0-3 months are males [3]. The incidence of malformations among neonates with UTI is similar in prematures and neonates. Nevertheless, prematures have a higher risk to suffer from UTI, as a result of their relatively low immunity state anda frequent use of invasive instruments, such as urinary catheters.
The clinical manifestations of UTI among neonates are non- specific [4]. These manifestations include:
1. Fever (20%-40%).
2. Failure to thrive (15%-43%).
3. Neonatal jaundice (3%-41%)-the hyperbilirubinemia is usually direct and related to cholestasis.
4. Diarrhea (3%-5%).
5. Feeding difficulties (3%-5%).
6. In prematures, additional manifestations might be observed: apnea and bradycardia (45%), dyspnea (30%), fatigue (30%) and hypoxia (12%).
The initial laboratory tests that have been carried out among neonates with signs and symptoms of UTI include:
1. Complete blood count – with the emphasis on white blood cells count and differential.
2. Urinalysis (dipstick and specific gravity) are not sensitive or specific for the diagnosis of UTI when done alone. Therefore, the diagnosis is based on a positive urinary culture.
3. Urinary culture – the sample must be taken by suprapubic aspiration (SPA) or bladder catheterization.
In the medical literature, there is a recommendation to perform radiologic examination for any neonate with UTI, due to the higher incidence of urinary system malformations. It includes renal and urinary tract US, cystography and finally renal scan [5,6].
After cultures are taken (urinary, blood and CSF if indicated), IV broad spectrum antibiotic treatment should be administrated without any delay. The antibiotic therapy includes both empirical treatment, which doesn't depend on the bacteria and following specific treatment, which is directed to the growing bacteria and their sensitivity (given with culture results). The urinary culture should become sterile within following 48 hours from the beginning of specific antibiotic treatment, directed to the pathogenic bacteria. As a result, the duration of the treatment is based on clinical judgment and experience. The common range for the treatment duration in neonates with uncomplicated UTI is 10-14 days [7-9].
Clinical relevance
Participants UTI among neonates might be very challenging for diagnosis, due to a unique epidemiology, varied, not specific signs and symptoms along with insensitive and not specific initial laboratory findings. An early detection of an infective process is necessary for an effective urgent treatment and prevention of severe disease complications. In a majority of cases, the infection occurs on the background of urinary tract congenital anomalies, whose recognition is also very important for a patient’s better prognosis. It was found that males are at risk of 2.5 times as compared to females. E. coli is the main pathogen that has been observed in this specific study. Tests, such as complete blood count, blood culture and dipstick weren't effective in terms of specificity and sensitivity for a diagnosis of UTI. 4% of neonates participating in the study suffered from urosepsis and most of them had urinary tract congenital anomalies that were detected by ultrasound. Researchers’ conclusions were that a urine culture must be taken in all neonates with high fever. To identify UTI a urinary tract ultrasound should be performed.
Due to all the factors mentioned above, there is an increasing need to investigate the process of neonatal UTI in our region in several aspects, such as epidemiology, microbiology of pathogens, clinical manifestations, blood and urine laboratory findings, imaging findings, treatment and prognosis. It's also important to compare the above written data with the other age groups for one year.
WIn this retrospective clinical study, there have been investigated the medical files of children up to 1 year of age hospitalized in the pediatric department of Baruch Padeh medical center in Poriya, between the years of 2011-2016, due to UTI.
The patients have been divided into 3 age groups:
1. First group: 0-1 month.
2. Second group: 1-3 months.
3. Third group: 3-12 months.
Investigated issues
• A connection between UTI and circumcision in males up to 1 month old.
• Time following circumcision when the clinical and laboratory signs of inflammation appeared.
• The difference in bacterial species among the different age groups.
• The connection between bacteria species and pathological US findings.
• Hospitalization period as a derivative of bacteria species, its sensitivity for antibiotics and renal US findings.
• The treatment during the hospitalization.
• Age and gender.
In terms of comparison between the different age groups:
• Identification of epidemiological difference among children with UTI in the different age groups.
• Identification of common bacteria in each age group, considering the status of resistance to typical antibiotic therapy, in a manner which allows choosing appropriate treatment.
• Identification of some typical symptoms and signs for UTI among the different age groups.
• Identification of the typical blood and urine examinations findings among the different age groups.
• Identification of the structural urinary tract pathologies and pathological findings in urinary tract imaging among the different age groups.
• Identification of the UTI outcomes in each age group in a way, which allows determining an appropriate follow up.
The incidence of UTI and the association to the other investigated issues will be tested using the Paired t-test.
This study covered 222 infants up to 1 year old, out of which there were 73.1% females and 26.9% males (43 circumcised and 17 noncircumcised). (Tables 1-8, Figures 1 and 2). There is a difficulty in determining an exclusive prognosis of UTI in neonates, since in a majority of cases there is an involvement of many other factors, such as preexisting kidney disease or other pathologies in urinary system (Tables 9-14 and Figures 3-6).
| Age group | Variable | Categories | Circumcised | Uncircumcised |
|---|---|---|---|---|
| <1 month | Hydronephrosis | Yes | 3 | 12 |
| No | 1 | 12 | ||
| Bacteria | E-coli | 3 | 19 | |
| Enterococous | 1 | 4 | ||
| Klebsiella pnemonia | 1 | 0 | ||
| Pseudomonas | 0 | 0 | ||
| Staphyloccous | 0 | 1 | ||
| Proteus mirablis | 0 | 0 | ||
| Enterobacter cloacae | 0 | 0 | ||
| Klebsiella oxytoca | 0 | 0 | ||
| 1-3 months | Hydronephrosis | Yes | 4 | 2 |
| No | 2 | 9 | ||
| Bacteria | E-coli | 4 | 9 | |
| Enterococous | 1 | 1 | ||
| Klebsiella pnemonia | 0 | 1 | ||
| Pseudomonas | 0 | 0 | ||
| Staphyloccous | 1 | 0 | ||
| Proteus mirablis | 0 | 0 | ||
| Enterobacter cloacae | 1 | 0 | ||
| Klebsiella oxytoca | 0 | 0 | ||
| 3-12 months | Hydronephrosis | Yes | 2 | 2 |
| No | 3 | 3 | ||
| Bacteria | E-coli | 4 | 3 | |
| Enterococous | 1 | 2 | ||
| Klebsiella pnemonia | 0 | 1 | ||
| Pseudomonas | 0 | 0 | ||
| Staphyloccous | 0 | 0 | ||
| Proteus mirablis | 0 | 0 | ||
| Enterobacter cloacae | 0 | 0 | ||
| Klebsiella oxytoca | 0 | 1 |
Table 1: The following tables present some essential data regarding the background features (in percentages).
| Prevalence (n=222) | ||
|---|---|---|
| <1 month | 29 | |
| Age group | 1-3 months | 36 |
| 3-12 months | 157 | |
| Fever | 215 | |
| Seizure | 1 | |
| Indications of hospitalization | Afebrile illness | 3 |
| Restlessness | 2 | |
| Failure to thrive | 2 |
Note: The clinical manifestation in all infants was a high fever without age references. The restlessness was more prominent in infants. In infants with UTI and a history of vesicoureteral reflux, the clinical manifestation was a failure to thrive
Table 2: Age group hospitalizations.
| Variable | Category | <1 month | 1 month–3 months | 3 months–12 months |
|---|---|---|---|---|
| ESBL | Yes (quantity) | 0 | 0 | 4 |
| Yes (percentage) | 0 | 0 | 100 | |
| No (quantity) | 28 | 31 | 131 | |
| Bacteria | E-coli | 22 | 27 | 134 |
| Enterococous | 2 | 2 | 7 | |
| Klebsiella pnemonia | 1 | 4 | 5 | |
| Pseudomonas | 0 | 0 | 7 | |
| Staphyloccous | 2 | 1 | 0 | |
| Proteus mirablis | 0 | 1 | 3 | |
| Enterobacter cloacae | 0 | 1 | 0 | |
| Klebsiella oxytoca | 0 | 0 | 1 | |
| Augmentin | S | 23 | 30 | 109 |
| I | 1 | 2 | 14 | |
| R | 5 | 4 | 33 | |
| Resprim | S | 21 | 24 | 115 |
| I | 2 | 3 | 3 | |
| R | 6 | 9 | 37 | |
| 1st generation cephalosporines | S | 25 | 34 | 127 |
| I | 1 | 1 | 6 | |
| R | 3 | 1 | 3 | |
| 3rd generation cephaosporines | S | 28 | 35 | 216 |
| I | 0 | 1 | 4 | |
| R | 0 | 0 | 1 |
Note: In a majority of cases the renal ultrasound was normal, with no evidence of hydronephrosis. This finding excludes the presence of vesicoureteral reflux (VUR). Among the male infants, the infection was in proximity to the circumcision, especially with E.coli.
Table 3: Presence of VUR
| Females | Males | Categories | Variable | Age group |
|---|---|---|---|---|
| 0 | 22 | E-coli | Bacteria | <1 month |
| 0 | 5 | Enterococous | ||
| 0 | 1 | Klebsiella pnemonia | ||
| 0 | 0 | Pseudomonas | ||
| 0 | 1 | Staphyloccous | ||
| 0 | 0 | Proteus mirablis | ||
| 0 | 0 | Enterobacter cloacae | ||
| 0 | 0 | Klebsiella oxytoca | ||
| 14 | 13 | E-coli | Bacteria | 1-3 months |
| 0 | 2 | Enterococous | ||
| 3 | 1 | Klebsiella pnemonia | ||
| 0 | 0 | Pseudomonas | ||
| 0 | 1 | Staphyloccous | ||
| 1 | 0 | Proteus mirablis | ||
| 0 | 1 | Enterobacter cloacae | ||
| 0 | 0 | Klebsiella oxytoca | ||
| 127 | 7 | E-coli | Bacteria | 3-12 months |
| 4 | 3 | Enterococous | ||
| 4 | 1 | Klebsiella pnemonia | ||
| 7 | 0 | Pseudomonas | ||
| 0 | 0 | Staphyloccous | ||
| 3 | 0 | Proteus mirablis | ||
| 0 | 0 | Enterobacter cloacae | ||
| 0 | 1 | Klebsiella oxytoca |
Note: Urinary tract infection UTI follow-up-The most common pathogen was E-coli, followed by Enterococcus, especially among young infants. Presence of Enterobacter cloacae was rare.
Table 4: Urinary tract infection followup.
| Age group | Variable | Categories | |
|---|---|---|---|
| Clinical manifestation | Number of infants with fever | 26 | |
| Number of infants without fever | 3 | ||
| WBC | N | 29 | |
| Mean | 13.84 | ||
| std | 5.45 | ||
| Neutrophils Abs | N | 29 | |
| Mean | 7.23 | ||
| std | 4.26 | ||
| <1 month | Neutrophils pct | N | 29 |
| Mean | 50.14 | ||
| std | 15.05 | ||
| ESR | N | 21 | |
| Mean | 65.19 | ||
| std | 20.76 | ||
| CRP | N | 13 | |
| Mean | 69.15 | ||
| std | 33.186 | ||
| Clinical manifestation | Number of infants with fever | 36 | |
| Number of infants without fever | 0 | ||
| WBC | N | 36 | |
| Mean | 12.67 | ||
| std | 4.81 | ||
| Neutrophils Abs | N | 36 | |
| Mean | 6.65 | ||
| std | 3.5 | ||
| 1-3 months | Neutrophils pct | N | 36 |
| Mean | 50.58 | ||
| std | 15.57 | ||
| ESR | N | 25 | |
| Mean | 53.08 | ||
| std | 19.81 | ||
| CRP | N | 18 | |
| Mean | 63.49 | ||
| std | 29.156 | ||
| Clinical manifestation | Number of infants with fever | 152 | |
| Number of infants without fever | 5 | ||
| WBC | N | 156 | |
| Mean | 17.48 | ||
| std | 6.69 | ||
| Neutrophils Abs | N | 157 | |
| Mean | 10.35 | ||
| std | 6.16 | ||
| 3-12 months | Neutrophils pct | N | 157 |
| Mean | 55.64 | ||
| std | 14.86 | ||
| ESR | N | 131 | |
| Mean | 67.46 | ||
| std | 25.08 | ||
| CRP | N | 87 | |
| Mean | 82.60 | ||
| std | 24.92 |
Note: 1. In ages 3-12 months we observed an increase in ESR and CRP. 3. Leukocytosis and neutrophilia were not prominent. 3. In ages of <3 months the CRP values were not high, and the ESR values were not.
Table 5: Observed increase in ESR and CRP range in ages between 3-12 months.
| Gender | Total | ||||
|---|---|---|---|---|---|
| Male | Female | ||||
| 1m | Count | 29 | 0 | 29 | |
| % within age group | 100.0% | 0.0% | 100.0% | ||
| age group | 1-3m | Count | 18 | 18 | 36 |
| % within age group | 50.0% | 50.0% | 100.0% | ||
| 3-12m | Count | 12 | 145 | 157 | |
| % within age group | 7.6% | 92.4% | 100.0% | ||
| Total | Count | 59 | 163 | 222 | |
| % within age group | 26.6% | 73.4% | 100.0% |
Note: 1. According the table, the incidence of infection among neonates was higher in males, reaching 100%. 2. In ages 1-3 months, the ratio between males and females became equal, 50% males and 50% females. 3. In ages 3-12 months the incidence of infection was much higher in females in compare to males, 93% females and 7% males.
Table 6: Observed increase in ESR and CRP range in ages between 3-12 months.
| Crosstable | ||||
|---|---|---|---|---|
| Urine leukocytes N | Y | Total | ||
| E-coli | Count | 20 | 160 | 180 |
| % within urine culture | 11.10% | 88.90% | 100.00% | |
| Enterococous | Count | 7 | 8 | 15 |
| % within urine culture | 46.70% | 53.30% | 100.00% | |
| Kleniellr pneumonia | Count | 2 | 8 | 10 |
| % within urine culture | 20.00% | 80.00% | 100.00% | |
| Pseudomonas | Count | 1 | 6 | 7 |
| % within urine culture | 14.30% | 85.70% | 100.00% | |
| Urine culture | ||||
| Count | 0 | 2 | 2 | |
| Staphyloccous | ||||
| % within urine culture | 0.00% | 100.00% | 100.00% | |
| Proteus mirablis | Count | 0 | 4 | 4 |
| % within urine culture | 0.00% | 100.00% | 100.00% | |
| Enterobacter cloacae | Count | 1 | 0 | 1 |
| % within urine culture | 100.00% | 0.00% | 100.00% | |
| Klebsiela oxytoca | Count | 0 | 1 | 1 |
| % within urine culture | 0.00% | 100.00% | 100.00% | |
| Total | Count | 31 | 189 | 220 |
| % within urine culture | 14.10% | 85.90% | 100.00% | |
Note: 1. The level of leukocytosis did not correlate with the bacterial species. 2. E.coli manifested a higher level of leukocyturia. 3. Pathogens, such as Klebsiella and Pseudomonas showed a lower level of leukocyturia.
Table 7: Crosstable of urine culture.
| Crosstable | ||||
|---|---|---|---|---|
| Urine nitrates | Total | |||
| N | Y | |||
| E-coli | Count | 117 | 65 | 182 |
| % within urine culture | 64.30% | 35.70% | 100.00% | |
| Enterococous | Count | 12 | 3 | 15 |
| % within urine culture | 80.00% | 20.00% | 100.00% | |
| Kleniellr pneumonia | Count | 6 | 4 | 10 |
| % within urine culture | 60.00% | 40.00% | 100.00% | |
| Pseudomonas | Count | 6 | 1 | 7 |
| % within urine culture | 85.70% | 14.30% | 100.00% | |
| Urine culture | ||||
| Count | 2 | 0 | 2 | |
| Staphyloccous | ||||
| % within urine culture | 100.00% | 0.00% | 100.00% | |
| Proteus mirablis | Count | 3 | 1 | 4 |
| % within urine culture | 75.00% | 25.00% | 100.00% | |
| Enterobacter cloacae | Count | 1 | 0 | 1 |
| % within Urine Culture | 100.00% | 0.00% | 100.00% | |
| Klebsiela oxytoca | Count | 1 | 0 | 1 |
| % within urine culture | 100.00% | 0.00% | 100.00% | |
Note: The presence of nitrites in urinalysis was not significant in the diagnosis of the growing bacteria, especially with gram negative bacteria. It is related to several factors, such as infant’s nutrition and the reliability of the dipstick.
Table 8: Presence of nitrites in urinalysis.
| age group | Total | |||||
|---|---|---|---|---|---|---|
| 1m=0 | 1-3 m=1 | 3-12 m=2 | ||||
| ESBL | N | Count | 28 | 31 | 131 | 190 |
| % within | 14.70% | 16.30 | 68.90% | 100.00 | ||
| ESBL | % | % | ||||
| Y | Count | 0 | 0 | 4 | 4 | |
| % within | 0.00% | 0.00% | 100.00 | 100.00 | ||
| ESBL | % | % | ||||
| Total | Count | 28 | 31 | 135 | 194 | |
| % within | 14.40% | 16.00 | 69.60% | 100.00 | ||
| ESBL | % | % |
Table 9: ESBL analysis.
| Crosstab | |||||
|---|---|---|---|---|---|
| age group | Total | ||||
| 1 m=0 | 1-3 m=1 | 3-12m=2 | |||
E-coli |
Count | 22 | 27 | 134 | 183 |
| Enterococous | % within urine culture | 12.00% | 14.80% | 73.20% | 100.00% |
| Count | 5 | 2 | 7 | 14 | |
|
Kleniellr pnemonia |
% within urine culture | 35.70% | 14.30% | 50.00% | 100.00% |
| Count | 1 | 4 | 5 | 10 | |
| Pseudomonas | % within urine culture | 10.00% | 40.00% | 50.00% | 100.00% |
| Count | 0 | 0 | 7 | 7 | |
| % within urine culture | 0.00% | 0.00% | 100.00% | 100.00% | |
| Urine culture | |||||
| Count | 1 | 1 | 0 | 2 | |
| Staphyloccous | % within urine culture | 50.00% | 50.00% | 0.00% | 100.00% |
| Count | 0 | 1 | 3 | 4 | |
Proteus mirablis |
% within urine culture | 0.00% | 25.00% | 75.00% | 100.00% |
| Count | 0 | 1 | 0 | 1 | |
Enterobacter cloacae |
% within urine culture | 0.00% | 100.00% | 0.00% | 100.00% |
| Count | 0 | 0 | 1 | 1 | |
Klebsiela oxytoca |
% within urine culture | 0.00% | 0.00% | 100.00% | 100.00% |
| Count | 29 | 36 | 157 | 222 | |
| Total | % within urine culture | 13.10% | 16.20% | 70.70% | 100.00% |
Table 10: Bacterial count in urine culture samples.
| Crosstab |
||||||
|---|---|---|---|---|---|---|
| Age Group | Total | |||||
| Augmentin | S | Count | 1 m=0 | 1-3 m=1 | 3-12 m=2 | |
| 23 | 30 | 109 | 162 | |||
| % within augmentin | 14.20% | 18.50% | 67.30% | 100.00% | ||
| I | Count | 1 | 2 | 14 | 17 | |
| % within augmentin | 5.90% | 11.80% | 82.40% | 100.00% | ||
| R | Count | 5 | 4 | 33 | 42 | |
| % within augmentin | 11.90% | 9.50% | 78.60% | 100.00% | ||
| Total | Count | 29 | 36 | 156 | 221 | |
| % within augmentin | 13.10% | 16.30% | 70.60% | 100.00% | ||
Table 11: Sensitivity to augmentin.
| Crosstab | ||||||
|---|---|---|---|---|---|---|
| age group | Total | |||||
| 1 m=0 | 1-3 m=1 | 3-12 m=2 | ||||
| S | Count | 21 | 24 | 115 | 160 | |
| % within RESPRIM | 13.10% | 15.00% | 71.90% | 100.00% | ||
| RESPRIM | I | Count | 2 | 3 | 3 | 8 |
| % within RESPRIM | 25.00% | 37.50% | 37.50% | 100.00% | ||
| R | Count | 6 | 9 | 37 | 52 | |
| % within RESPRIM | 11.50% | 17.30% | 71.20% | 100.00% | ||
| Ttal | Count | 29 | 36 | 155 | 220 | |
| % within RESPRIM | 13.20% | 16.40% | 70.50% | 100.00% |
Table 12: RESPRIM analysis.
| Crosstab | ||||||
|---|---|---|---|---|---|---|
| age group | Total | |||||
| 1 m=0 | 1-3 m=1 | 3-12 m=2 | ||||
| S | Count | 25 | 34 | 127 | 186 | |
| % within CEPH_1g | 13.40% | 18.30% | 68.30% | 100.00% | ||
| CEPH_1g | I | Count | 1 | 1 | 6 | 8 |
| % within CEPH_1g | 12.50% | 12.50% | 75.00% | 100.00% | ||
| R | Count | 3 | 1 | 23 | 27 | |
| % within CEPH_1g | 11.10% | 3.70% | 85.20% | 100.00% | ||
| Ttal | Count | 29 | 36 | 156 | 221 | |
| % within CEPH_1g | 13.10% | 16.30% | 70.60% | 100.00% |
Note: Resistance to 1st generation Cephalosporin was documented in 27 cases, especially in ages of 3-12 months. Moderate resistance was found in the age group of 1-3 month, while mild resistance was found under the age of 1 month.
Table 13: Resistance to 1st generation cephalosporin.
| Crosstab | ||||||
|---|---|---|---|---|---|---|
| age group | Total | |||||
| 1m=0 | 1-3m=1 | 3-12m=2 | ||||
| CEPH_3g | S | Count | 28 | 35 | 153 | 216 |
| % within CEPH_3g | 13.00% | 16.20% | 70.80% | 100.00% | ||
| R | Count | 0 | 1 | 3 | 4 | |
| % within CEPH_3g | 0.00% | 25.00% | 75.00% | 100.00% | ||
| 3 | Count | 0 | 0 | 1 | 1 | |
| % within CEPH_3g | 0.00% | 0.00% | 100.00% | 100.00% | ||
| Total | Count | 28 | 36 | 157 | 221 | |
| % within CEPH_3g | 12.70% | 16.30% | 71.00% | 100.00% | ||
Note: No resistance to 3rd generation Cephalosporin was documented among neonates.3 cases of resistance were documented in the group age of 3-12 months. 1 case of resistance was documented in the group age of 1-3 months Figure 2-5
Table 14: Third generation cephalosporin.
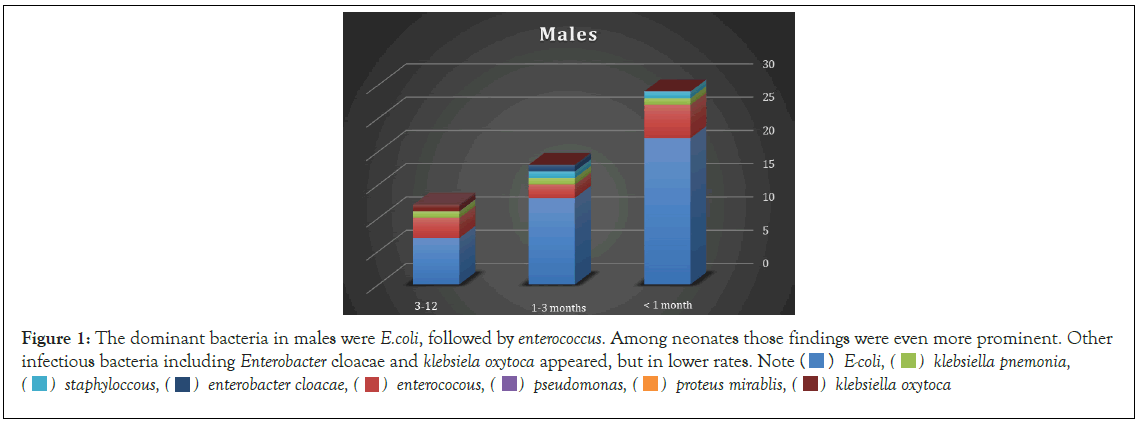
Figure 1: The dominant bacteria in males were E.coli, followed by enterococcus. Among neonates those findings were even more prominent. Other infectious bacteria including Enterobacter cloacae and Klebsiela oxytoca appeared, but in lower rates. Note 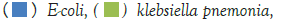
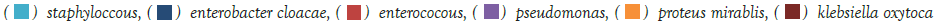
Figure 2: Under the age of 1 month, no specific bacteria were found to be dominant in causing UTI. In the ages 1-3 months, the leading bacteria causing UTI was E.coli, and in rare cases Klebsiella. E.coli was the most dominant bacteria in the age group of 3-12 months. 

Figure 3: RESPRIM. Note: 
Figure 4: During younger ages, the sensitivity to Augmentin was higher in comparison to Resprim. However, as the age increased, the sensitivity to Resprim became higher than Augmentin.
Figure 5: The sensitivity was lower among younger ages. Nevertheless, it became higher as the infant age increased. It is associated with the dominant bacteria–E. coli. 
Figure 6: The sensitivity is very high, with almost no resistance in neonatal period. Minor resistances were documented in older age groups. 
Evidence based medicine confirms that the risk for renal scaring, which results from UTI is much higher under one year of age, in comparison to older ages.
In addition, neonates with Vesicoureteral Reflux (VUR) have a higher risk to suffer from renal scaring. Renal scaring increases the risk for hypertension and chronic renal diseases. It’s indicated that about 4 years following the infection’ kidney growth decreased, regardless the presence of VUR. However, the final kidney size is not smaller in the end of the whole growing process.
The aim of our study was to check the clinical and microbiologic characteristics of infants diagnosed with first UTI episode, with special emphasis on the characteristics of the first recurrent UTI episode. Our results after analyzing the clinical and microbiological causes showed: the most common isolated pathogens were E. coli, Klebsiella spp., P. mirabilis, Enterococcus spp., and S. aureus. In the recurrent infections these bacteria were observed more resistance especially to Aminoglycosides and Tazocin. The resistance rates of E. coli and Klebsiella spp. isolates were high for ampicillin, amoxicillin/clavulanic acid, TMP/SMX, cefuroxime, ceftriaxone and gentamicin, moderate for piperacillin/tazobactam and low for ciprofloxacin, amikacin and meropenem. High ESBL production rates were recorded among E. coli and Klebsiella spp. isolates; and no differences were recorded between the initial and recurrent UTI episodes in the resistance rates of E. coli and Klebsiella spp., except for increased resistance rates of both pathogens to piperacillin/ tazobactam at the recurrent episode.
In conclusion, Recurrent UTIs were characterized by different uropathogens and increased antibiotic resistance. Our findings make the choice of the appropriate antibiotic treatment for UTI more challenging, and our study emphasizes the need for tight epidemiologic follow-up and active, efficacious antibiotic administration.
Citation: Nasser H, Nasser E, Nasser S, Sigal S, Goldshtein S, Micheal J, et al. (2021) Clinical and Laboratory Approach of Urinary Tract Infection in Infants. Clin Pediatr. 6:197.
Received: 11-Nov-2021 Accepted: 25-Nov-2021 Published: 02-Dec-2021
Copyright: © Nasser H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.