
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2020)Volume 11, Issue 4
Background: Since December 2019, pneumonia caused by a novel coronavirus (SARS-CoV-2), namely COVID-19,
has rapidly spread from Wuhan city to other cities across China, with the cumulative number of infections
reaching 80,000. According to official data from the Chinese Center for Disease Control and Prevention, clinical
characteristics of COVID-19 patients in Wuhan are significantly different from COVID-19 patients in other cities.
Objective: To describe the epidemiology, clinical characteristics, treatment, and prognosis of 74 hospitalized patients
with COVID-19.
Design: Retrospective, single-center case study.
Methods: Clinical data of 74 COVID-19 patients discharged from the Anhui Provincial Hospital Infectious
Disease Hospital (Hefei city, Anhui Province) from January 21 to February 25, 2020, were collected to analyze the
epidemiological, demographics, laboratory, radiological, and treatment data. Thirty-two patients were followed up
and tested for the presence of the viral nucleic acid and by pulmonary computed tomography (CT) scan at 7 and 14
days after they were discharged.
Results: Among all COVID-19 patients, 60% were young adults (19–65 years), with more males than females.
Thirty-six patients had a history of close contact with people from Wuhan two weeks before the disease onset,
accounting for 49% of the total. The median incubation period for patients was 6 days; the median period from
symptom onset to admission was 6 days, and the median length of hospital stay was 13 days. Fever symptoms were
presented in 84% of the patients, and the second most common symptom was cough (74%), followed by fatigue and
expectoration (27%). Lymphopenia occurred in 46% of the patients and was common (61%) in the patients in the
intensive care unit (ICU). Inflammatory indicators, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP),
and interleukin (IL)-6 of the ICU patients were significantly higher than that of the non-ICU patients. However,
50% of the patients had their CD4/CD8 ratio lower than 1.1. The CT results showed no signs of pneumonia in 8%
(six cases) of the patients and unilateral and bilateral involvements in 22% and 70% of the patients, respectively.
Antiviral therapy was used to treat 97% of patients (oral administration of lopinavir and ritonavir tablets), 81%
received antibiotic prevention or treatment, 22% interferon nebulization, and relatively few patients received steroid
and gamma globulin pulse therapies. Eighty-three percent of ICU patients inhaled high-flow oxygen and did not
receive invasive ventilation. One patient died of acute cerebral infarction accompanied by cerebral herniation and
had ground-glass opacities in the lung and positive viral nucleic acid testing during hospitalization. Thirty-two
patients received initial follow-up, and two of them had positive viral nucleic acids in the retests but no reinfection
signs.
Conclusion: Approximately half of the COVID-19 patients in our hospital had a history of close contact with people
from Wuhan. Fever, cough, expectoration, and fatigue were the most common symptoms. Compared with patients
in Wuhan, COVID-19 patients in Anhui Province had milder conditions and optimistic therapeutic outcomes,
suggesting that there may be some regional differences in the transmission of SARS-CoV-2 between different cities.
2019 novel coronavirus disease (COVID-19); Severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2); Pneumonia; Epidemiological characteristics; Treatment; Prognosis
In early December 2019, mysterious pneumonia was first discovered in Wuhan, Hubei Province. High-throughput sequencing revealed a novel coronavirus, initially named as 2019-nCoV, which had not been found in humans or animals previously [1]. Due to similarities with the early clinical symptoms of infection by severe acute respiratory syndrome coronavirus (SARS-CoV), the International Committee on Taxonomy of Virus named this novel coronavirus as SARS-CoV-2, and the World Health Organization (WHO) named the disease caused by SARS-CoV-2 as coronavirus disease 2019 (COVID-19) [2].
A study from the European Center for Disease Prevention and Control showed that Middle East respiratory syndrome coronavirus (MERS-CoV) infection had mortalities of 41.1% in the Middle East, 53.3% in Europe, 40.0% in Africa, and 19.2% in Asia, showing a significant regional difference [3]. This difference is difficult to explain with viral mutations [4]. With the significant domestic travel period due to the Spring Festival in 2020, SARS- CoV-2 began to spread across China. According to the official data released by the Chinese Center for Disease Control and Prevention [5], there are differences in the mortality and cure rates between provinces and cities in China. A study of Huang et al. [3] reported for the first time the clinical characteristics (including fever, dry cough, dyspnea, myalgia, fatigue, normal or decreased white blood cell count, and radiographic findings of pneumonia) and complications (29% respiratory distress syndrome, 12% heart injury, and 15% mortality) in 41 COVID-19 patients in Wuhan (28 discharged cases). However, Xiao et al. conducted a retrospective analysis of clinical characteristics of 62 COVID-19 patients in Zhejiang Province hospitalized within 16 days and found that most patients in Zhejiang Province had mild to moderate symptoms. In addition, only a small portion of the patients had dyspnea, and one of them developed respiratory distress syndrome who required intensive care unit (ICU) treatment. These researchers believe that the symptoms of patients diagnosed outside Wuhan are relatively mild. However, the analysis was based on the clinical data of 61 COVID-19 patients during the time of hospitalization (only one was discharged), and information on cure and complications is lacking because of the short study duration. The conclusion drawn may thus be limited. Anhui Province reported the first case of COVID-19 on January 21, 2020. As of February 25, 2020, the province had 989 confirmed cases, and no new cases were reported for four consecutive days. Given the literature research findings that COVID-19 patients in Wuhan and cities outside Wuhan show inconsistencies, we conducted a retrospective analysis on the epidemiological and clinical characteristics of 74 cured COVID-19 patients in Hefei city, Anhui Province to provide our clinical treatment data to cities affected by COVID-19.
As a provincial designated hospital, Anhui Provincial Hospital Infectious Disease Hospital (Hefei city, Anhui Province) is one of the four major bases for treating severely ill patients with COVID-19. This retrospective study included COVID-19 patients hospitalized in Anhui Provincial Hospital Infectious Disease Hospital from January 21, 2020, to February 25, 2020. A real- time reverse transcription-polymerase chain reaction was adopted as a diagnostic method of testing SARS-CoV-2 nucleic acids in nasopharyngeal swabs. Patients who had two positive tested results within 24 h were confirmed with COVID-19. The specific diagnosis and treatment processes of all patients in this study were carried out with reference to the guidelines of the WHO [6], and the treatment plans for severe and critically ill patients were submitted to the provincial expert group for discussion and decision. Epidemiological characteristics, clinical symptoms and signs, laboratory test results, and treatment during hospitalization were extracted from the electronic medical records by three trained clinicians. Epidemiological data were collected through a brief interview with each patient, and the patient’s contact history for the two weeks before the disease onset was collected, including the date and time of close contact (meeting, gathering, or co-working) with people from Wuhan or patients confirmed with COVID-19. Laboratory assessments included complete blood count, T-cell differential counts, blood chemistry, coagulation tests, liver and kidney function, electrolytes, C-reaction protein, procalcitonin, lactate dehydrogenase, and creatine kinase tests. Radiological assessment was mainly a pulmonary CT scan. Treatment measures included antiviral therapy, antibiotic therapy, corticosteroid therapy, interferon, gamma globulin pulse therapies, and high-flow oxygen therapy.
The endpoint of this study was discharge or death. Discharge criteria were normal body temperature for more than three days, significantly improved respiratory symptoms, imaging showing significant improvement in exudative changes, and two consecutive negative viral nucleic acid testing results in respiratory specimens (intervals>24 h). Patients who had been discharged were followed up at 7 and 14 days after discharge.
This study was approved by the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China. Given the urgency in collecting clinical data, the signing of written informed consent by the patients was waived.
Statistical analysis
In this study, categorical variables were presented as percentages, and continuous variables were presented as the median and interquartile range (IQR). The severity of admitted patients was evaluated according to the guidelines described in the literature [7]. Data of ICU and non-ICU patients were separated. SPSS 20.0 software (IBM Inc., Armonk, NY) was used for statistical analysis.
As of February 25, 2020, this study included data of clinical symptoms and outcomes of 74 COVID-19 patients, accounting for 87% (74/85) of patients admitted to infectious disease hospitals in Hefei city. A total of 15 patients were discharged and completed the follow-up examinations, and all patients underwent viral nucleic acid testing and pulmonary CT scan.
The demographic and clinical characteristics of patients in Table 1 showed that 60% of the COVID-19 patients in this study were young adults (19–65 years old), and the majority were males. Thirty-six COVID-19 patients (49%) had a history of close contact with people from Wuhan two weeks before the disease onset and were mainly imported COVID-19 cases. Seventeen patients had history of contact with confirmed COVID-19 patients, accounting for 23%. In addition, 28% of the patients did not have a history of contact with people from Wuhan or with confirmed COVID-19 patients. Twenty-one patients were in familial clusters of developing COVID-19, accounting for 28%. Among our patients, the median incubation period was 6 days, the median period from symptom onset to admission was 6 days, and the median length of hospital stay was 13 days. On admission, 84% of patients had fever, and the second most common symptom was dry cough (74%), followed by fatigue and expectoration (27%), headache (22%), and the less common symptoms, such as diarrhea, sore throat, and hemoptysis. The median age of COVID-19 patients in ICU was 56 years, and 56% of them had two or more complications (e.g., hypertension and cardiovascular disease). The median age of non-ICU patients was 43 years, and 13% of them had two or more complications. The median time from the onset of symptoms to hospital admission was 9.5 days in ICU patients, which was longer than that of non-ICU patients (5 days). The incubation period and contact history of ICU and non-ICU patients were similar.
| Clinical characteristics | Cases (%) | ||
|---|---|---|---|
| Total (n=74) | ICU patient (n=18) | Non-ICU patient (n=56) | |
| Age, median age, years | 47 (35–56) | 56 (47–68) | 43 (29–52) |
| Age group (years) | |||
| ≤ 18 | 2 (3) | 0 (0) | 2 (4) |
| 19–40 | 25 (34) | 2 (11) | 23 (41) |
| 41–65 | 35 (47) | 10 (55) | 25 (45) |
| ≥ 66 | 12 (16) | 6 (33) | 6 (11) |
| Sex | |||
| Male | 48 (65) | 15 (83) | 33 (59) |
| Female | 26 (35) | 3 (17) | 23 (41) |
| Exposure within 2 weeks | |||
| People from Wuhan | 36 (49) | 8 (44) | 28 (50) |
| Contact with confirmed COVID-19 patients | 17 (23) | 6 (33) | 11 (20) |
| No | 21 (28) | 4 (22) | 17 (30) |
| Familial clustering | 21 (28) | 9 (50) | 12 (21) |
| Incubation period, median, days | 6 (4–10) | 6 (5–10) | 6 (4–10) |
| Common signs and symptoms | |||
| Fever | 62 (84) | 15 (83) | 47 (84) |
| Cough | 55 (74) | 10 (56) | 45 (80) |
| Fatigue | 20 (27) | 4 (22) | 16 (29) |
| Myalgia | 11 (15) | 8 (44) | 3 (5) |
| Difficulty breathing | 5 (7) | 5 (28) | 0 (0) |
| Sore throat | 7 (9) | 1 (6) | 6 (11) |
| Expectoration | 20 (27) | 3 (17) | 17 (30) |
| Diarrhoea | 5 (7) | 3 (17) | 2 (4) |
| Headache | 16 (22) | 2 (11) | 14 (25) |
| Hemoptysis | 1 (1) | 1 (6) | 0 (0) |
| Time from symptom onset to admission, median, days | 6 (4–10) | 9.5 (6–12) | 5 (4–8.25) |
| Signs upon admission | |||
| Body temperature (°C) | |||
| ≤ 37.2 | 43 (58) | 9 (50) | 34 (61) |
| 37.3–38.0 | 19 (26) | 5 (28) | 14 (25) |
| 38.1–39.0 | 10 (14) | 3 (17) | 7 (13) |
| ≥ 39.1 | 2 (3) | 1 (6) | 1 (2) |
| Respiratory rate, median, breath/min | 20 (19–20) | 19.5 (17.25–20) | 20 (19–20) |
| Pulse, median, beats/min | 85 (79–90) | 81.5 (76.5–89.5) | 85 (80–90.5) |
| Mean arterial pressure, median, mmHg | 92 (86.7–97.3) | 93.65 (87.8–100.9) | 92 (86.7–97) |
| Oxygen saturation (without oxygen therapy), % | 98 (95–98.8) | 95 (91–97.8) | 98 (96–99) |
| Length of hospital stay | 13 (10.3–16) | 16 (3–18.8) | 12 (9.8–15) |
| ≥ 2 underlying diseases | 17 (23) | 10 (56) | 7 (13) |
| Hypertension | 21 (28) | 8 (44) | 13 (18) |
| Cardiovascular disease | 18 (24) | 4 (22) | 14 (25) |
| Diabetes | 9 (12) | 1 (6) | 8 (14) |
| Chronic obstructive pulmonary disease (COPD) | 2 (3) | 1 (6) | 1 (2) |
| Chronic kidney disease | 5 (7) | 1 (6) | 4 (7) |
| Chronic liver disease | 8 (11) | 1 (6) | 7 (13) |
| Sequela of stroke | 4 (5) | 3 (17) | 1 (2) |
| Malignant tumor | 1 (1) | 1 (6) | 0 (0) |
Table 1: Clinical characteristics of 74 COVID-19 patients in this study.
In Table 2, the results of laboratory examinations performed at the time of admission showed that 46% of all patients included in this study had lymphopenia, which was more common in ICU patients (61%) than the non-ICU patients. ESR, CRP, and IL-6, among other inflammatory indicators, were significantly higher in the ICU patients compared to the non-ICU patients. However, 50% of the patients had their CD4/CD8 ratio lower than 1.1. The creatine kinase, lactate dehydrogenase, and troponin levels of the ICU patients were higher than those of the non-ICU patients. Alaina aminotransferases were higher in ICU patients than in non- ICU patients; however, albumin of ICU patients was lower than that of non-ICU patients.
| Items | Median (IQR) | Normal range | ||
|---|---|---|---|---|
| Total (n=74) | ICU Patient (n=18) | Non-ICU patient (n=56) | ||
| White blood cell count, × 109/L | 5.5 (4.4–6.6) | 5.9 (4.7–6.6) | 5.5 (4.1–6.6) | 3.5–9.5 |
| Neutrophil count, × 109/L | 3.8 (2.7–5.2) | 4.4 (3.4–5.7) | 3.7 (2.5–5.0) | 1.8–6.3 |
| Lymphocyte count, × 109/L | 1.0 (0.6–1.5) | 0.8 (0.5–1.1) | 1.0 (0.6–1.8) | 1.1–3.2 |
| <1.1 × 109/L, percentage | 34 (46) | 11 (61) | 23 (41) | |
| Monocyte count, × 109/L | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) | 0.3 (0.4–0.5) | 0.1–0.6 |
| Platelet count, × 109/L | 166 (129–213) | 136 (113–165) | 190 (141–224) | 125.0–350.0 |
| ESR (mm/h) | 15 (10–18) | 21 (16–42.5) | 14 (9.8–17.3) | 0–20.0 |
| CRP (mg/L) | 17.4 (4.2–49.3) | 43.3 (12.2–72) | 13.6 (3.9–36.3) | 0–8.0 |
| CD4/CD8 | 1.3 (1.0–1.8) | 1.2 (0.9–1.4) | 1.3 (1.1–1.8) (n=19) | 1.1–1.7 |
| <1.1, percentage | 19 (26) | 9 (50) | 10 (18) | |
| Procalcitonin, ng/mL | 0.3 (0.1–0.7) | 0.2 (0.1–1.4) | 0.4 (0.2–0.7) (n=23) | 0–0.5 |
| IL-6 (pg/ml) | 6.6 (4.5–8.3) | 7.3 (6.2–14.2) | 4.7 (3.3–7.5) (n=17) | <7 |
| Creatine kinase, U/L | 91.4 (53.2–150.1) | 126.6 (63.9–242.3) | 80.7 (50.2–119.3) | 22–269 |
| Creatine kinase isoenzyme, U/L | 11.5 (8.8–15.8) | 11.4 (9.3–15.8) | 11.5 (8.7–11.9) | 0–225 |
| Lactate dehydrogenase, U/L | 269 (191–323) | 290 (239–354.5) | 238 (169–285) | 125–250 |
| High-sensitivity troponin, pg/mL | 0.08 (0.06–0.11) | 0.09 (0.08–0.31) | 0.08 (0.06–0.10) | 0–0.3 |
| Alanine aminotransferase, U/L | 23.5 (16–36.5) | 29 (24.5–41.5) | 18.5 (15–35.5) | 9–50 |
| Aspartate aminotransferase, U/L | 28.5 (21–38) | 35 (28.3–41.8) | 29 (28–42) | 15–45 |
| Albumin, g/l | 43.6 (37.2–47.6) | 38.2 (349–43.4) | 45 (39.3–48.8) | 40–55 |
| Total bilirubin, mmol/ | 14.9 (11.2–21.1) | 13.9 (9.7–18.9) | 16.1 (11.8–22) | 3.4–21 |
| Creatinine, μmol/L | 68 (59.5–80) | 70.5 (61–80) | 68 (58–80) | 57–111 |
| Urea nitrogen, mmol/L | 4.3 (3.2–5.9) | 4.9 (3.2–6.4) | 4.1 (3.2–5.7) | 3.1–8.0 |
| Prothrombin time, s | 14.2 (13.5–15.1) | 14.2 (14.8–15.4) | 14 (13.1–14.6) | 9.5–14.5 |
| Activated partial thromboplastin time (s) | 38.7 (32.8–42.3) | 35.8 (30.4–39.4) | 39.5 (33.2–44.7) | 20.0–40.0 |
| D-dimer, mg/L | 0.3 (0.1–0.6) | 0.5 (0.2–0.6) | 0.2 (0.1–0.4) | 0–1.1 |
Table 2: Laboratory test results of the 74 COVID-19 patients.
In Table 3, the pulmonary imaging findings of the patients at admission showed that 6 out of 74 COVID-19 patients (8%) had no signs of pneumonia on pulmonary CT images, while 22% and 70% of the patients in this study had unilateral and bilateral pulmonary involvement, respectively. Pneumonia with lymphadenopathy or pleural effusion was rare in our patients. Sixty-two percent of the non-ICU patients had ground-glass opacity, and 67% of the ICU patients had bilateral diffuse ground-glass opacities accompanied by partial pulmonary consolidation.
| CT manifestation | Number of people (percentage) | ||
|---|---|---|---|
| Total (n=74) | ICU patient (n=18) | Non-ICU patient (n=56) | |
| Pneumonia | |||
| Negative | 6 (8) | 0 (0) | 6 (11) |
| Unilateral ground-glass opacities | 16 (22) | 2(11) | 14 (25) |
| Bilateral ground-glass opacities | 25 (34) | 4 (22) | 21 (37) |
| Bilateral diffuse ground-glass opacities accompanied by partial pulmonary consolidation | 27 (36) | 12 (67) | 15 (26) |
| Comorbidities | |||
| Mediastinal lymphadenopathy | 6 (8) | 4 (22) | 2 (4) |
| Pleural effusion | 5 (7) | 4 (22) | 1 (2) |
Table 3: Pulmonary CT results of the 74 COVID-19 patients.
As shown in Table 4, the analysis of treatment and prognosis of patients showed that, among all COVID-19 patients, 99% of the patients received antiviral therapy (oral administration of lopinavir and ritonavir tablets; only a four-year-old child did not receive this therapy), 81% had antibiotic prevention or treatment, 22% had interferon nebulization, and relatively few patients received steroid and gamma globulin pulse therapies. No patient received invasive ventilation, and 83% of the ICU patients received high-flow oxygen therapy. Seventy-three (98.6%) patients were cured and discharged, with the median hospital stay of 13 days. The ICU patients stayed four days longer in the hospital than the non-ICU patients. In addition, the ICU patients had a significantly higher incidence of complications than non-ICU patients. Four patients with acute respiratory distress syndrome received treatment in ICU. There was one case of death (1.4%), and this patient died of acute cerebral infarction accompanied by cerebral herniation and had ground- glass opacities in the pulmonary CT and positive viral nucleic acid testing during the hospitalization and neurological screening.
| Number of people (percentage) | |||
|---|---|---|---|
| Total (n=74) | ICU patient (n=18) | Non-ICU patient (n=56) | |
| Complication | |||
| Shock | 1 (1) | 1 (6) | 0 (0) |
| Myocardial injury | 3 (4) | 2 (11) | 1 (2) |
| Arrhythmia | 1 (1) | 1 (6) | 0 (0) |
| Liver damage | 5 (7) | 3 (17) | 2 (4) |
| Renal impairment | 3 (4) | 2 (11) | 1 (2) |
| Acute respiratory distress syndrome | 4 (5) | 4 (22) | 0 (0) |
| Treatment | |||
| Antiviral | 72 (97) | 18 (100) | 55 (98) |
| Antibiotic | 60 (81) | 18 (100) | 42 (75) |
| Glucocorticoid | 4 (5) | 2 (11) | 2 (4) |
| Interferon | 16 (22) | 12 (67) | 4 (7) |
| Gamma globulin | 15 (20) | 11 (61) | 4 (7) |
| Tocilizumab | 4 (5) | 4 (22) | 0 |
| Oxygen | |||
| High-flow | 15 (20) | 15 (83) | 0 (0) |
| Nasal cannula | 38 (51) | 3 (17) | 35 (63) |
| Invasive ventilation | 0 (0) | 0 (0) | 0 (0) |
| Number of viral nucleic acid testing until getting 2 consecutive negative results | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Deaths | 1 (1) | 1 (6) | 0 (0) |
Table 4: Complications and treatment of the 74 COVID-19 patients.
Table 5 summarizes and compares the treatment data of COVID-19 patients in our province with the data of COVID-19 patients in Wuhan, Jiangsu, and the whole country. As of February 25, 15 discharged patients had their viral nucleic acid testing and pulmonary CT scan repeated at the outpatient fever clinic or another local hospital, and one patient (6.7%) showed positive viral nucleic acid testing by the local center of disease control, followed by two consecutive negative results three days later. No sign of reinfection was found in that patient. The pulmonary CT results of the 15 patients in the follow-up showed further resolution and repairing of pulmonary lesions (Figure 1 for typical cases).
| Item | Number of people (percentage) | |||
|---|---|---|---|---|
| Anhui (n=74) | Wuhan (n=41) | Zhejiang (n=62) | Whole country (1099) | |
| Age, median, years | 47 (35–56) | 49 (41–58) | 41 (32–52) | 47 (35–58) |
| Sex, male (%) | 48 (65) | 30 (73) | 36 (58) | 637 (58) |
| Contact history | 53 (72) | - | 23 (37) | - |
| People from Wuhan or confirmed COVID-19 patient(s) | 53 (72) | - | 23 (37) | 676/1099 (62) |
| Wuhan seafood market or wildlife | 0 (0) | 27 (66) | 0 (0) | 13/687 (2) |
| No | 21 (28) | - | 39 (63) | 285/1099 (25.9) |
| Familial clustering | 21 (28) | - | 21 (34) | - |
| Incubation period, median, days | 6 (4–10) | 8 (5–13) | 4 (3–5) | 4 (2–7) |
| Fever | 62 (84) | 40 (98) | 48 (77) | 975 (89) |
| Cough | 55 (74) | 31 (76) | 50 (81) | 745 (68) |
| Fatigue | 31 (42) | 18 (44) | 32 (52) | 419 (38.1) |
| Expectoration | 20 (27) | 11 (28) | 35 (56) | 370 (33.7) |
| Difficulty breathing | 5 (7) | 22 (55) | 2 (4) | 205 (18.7) |
| Headache | 16 (22) | 3 (8) | 21 (34) | 150 (13.6) |
| Diarrhea | 5 (7) | 1 (3) | 3 (8) | 42 (3.8) |
| Hemoptysis | 1 (1) | 2 (5) | 2 (3) | 10 (0.9) |
| Symptom onset to admission, median, days | 6 (4–10) | 5 (1–8) | 2 (1–4) | 4 (2–7) |
| Any comorbidities | 34 (46) | 13 (32) | 20 (32) | 261 (23.7) |
| White blood cell count, median, × 109/L | 5.5 (4.4–6.6) | 6.2 (4.1–10.5) | 4.7 (3.5–5.9) | 4.7 (3.5–6.0) |
| Lymphocyte count, × 109/L | 1.0 (0.6–1.5) | 0.8 (0.6–1.1) | 1.0 (0.8–1.5) | 1.0 (0.7–1.3) |
| ESR, median, mm/h | 15 (10–18) | - | - | - |
| CRP, median, mg/L | 17.4 (4.2–49.3) | - | - | ≥10,481/793 |
| Procalcitonin, median, ng/mL | 0.3 (0.1–0.7) | 0.1 (0.1–0.1) | 0.04 (0.03–0.06) | ≥0.5, 35/633 |
| Creatine kinase, median, U/L | 91.4 (53.2–150.1) | 132 (62–139) | 65 (49–101) | ≥200, 90/657 |
| Lactate dehydrogenase, U/L | 269 (191–323) | 286 (242–408) | 205 (184–260) | ≥250, 277/675 |
| Alanine aminotransferase, median, U/L | 23.5 (16–36.5) | 32 (21–50) | 22 (14–34) | ≥40, 158/741 |
| Aspartate aminotransferase, median, U/L | 28.5 (21–38) | 34 (26–48) | 26 (20–32) | ≥40, 168/757 |
| Creatinine, μmol/L | 68 (59.5–80) | 139 (137–140) | 72 (61–84) | ≥133, 12/752 |
| D-dimer, median, mg/L | 0.3 (0.1–0.6) | 0.5 (0.3–1.3) | 0.2 (0.2–0.5) | ≥0.5, 260/560 |
| Pneumonia | 68 (92) | - | 61 (98) | 972 (92) |
| Bilateral involvement | 52 (70) | 40 (98) | 52 (84) | - |
| ICU treatment | 18 (24) | - | 1 (2) | 55 (5) |
| Antiviral | 72 (97) | 38 (93) | 55 (89) | 393 (36) |
| Antibiotic | 60 (81) | 41 (100) | 28 (45) | 638 (58) |
| Steroid | 4 (5) | 9 (22) | 16 (26) | 204 (19) |
| Gamma globulin | 15 (20) | - | - | 144 (13) |
| Interferon | 16 (22) | - | 28 (45) | - |
| Oxygen therapy | 53 (72) | 41 (100) | - | 454 (41) |
| Noninvasive ventilation | 53 (72) | 10 (24) | - | 56 (5.1) |
| Invasive ventilation | 0 (0) | 2 (5) | - | 25 (2) |
| ECO | 0 (0) | 2 (5) | - | 5 (0.5) |
| Length of hospital stay | 13 (10–16) | - | - | 12 (10–14) |
| Death | 1 (1) | 6 (15) | 0 (0) | 15 (1) |
| Discharged | 73 (99) | 28 (68) | 1 (2) | 55 (5) |
Table 5: Patient demographics in Anhui Province, Wuhan city, Zhejiang Province, and the whole country.
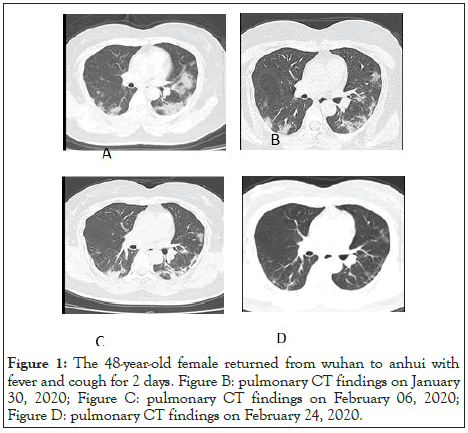
Figure 1: The 48-year-old female returned from wuhan to anhui with fever and cough for 2 days. Figure B: pulmonary CT findings on January 30, 2020; Figure C: pulmonary CT findings on February 06, 2020; Figure D: pulmonary CT findings on February 24, 2020.
Since December 2019, COVID-19 caused by SARS-CoV-2 has rapidly spread from Wuhan city (Hubei Province) to different provinces and cities across China. As of February 25, 2020, China has reported more than 70,000 confirmed cases of COVID-19, 989 of which have been reported in Anhui Province. As far as we know, this study is the retrospective report with the largest number of cured COVID-19 patients in Anhui Province so far, including a total of 74 COVID-19 patients discharged from hospital (2 critical, 25 severe, 41 moderate, and 6 mild cases). Of these, 15 patients were followed up for more than two weeks.
The minimum and maximum ages of the patients included in this study were 4 and 92 years old, respectively. Analysis according to the age group [8] suggested that 60% of our patients were young adults aged 19–65 years old, with the median age of 47 years, and 65% of our patients were male. These results were somewhat different from the findings reported earlier. Male COVID-19 patients in Wuhan accounted for 73% of the total number of patients (30 males of 41 COVID-19 patients), with a median age of 49 years; whereas in Jiangsu, 58% of the COVID-19 patients were male (36 males of 62 COVID-19 patients), with a median age of 41 years [3]. Approximately 50% of the COVID-19 patients in this study had close contact with people from Wuhan two weeks before the onset. Although they were mainly imported cases, they had no history of contact with the Huanan Seafood Market in Wuhan, which was different from the patients in Wuhan. Familial clustering of COVID-19 occurred in 21 patients, which was consistent with the previous reports of human-to-human transmission [8]. The most common symptoms of patients in this study at the time of hospital admission were fever (84%), dry cough (74%), fatigue (27%), and expectoration (27%), while dyspnea symptoms accounted for only 7%, which was significantly different from the COVID-19 patients in Wuhan (55% of the patients had dyspnea) [3]. Four of the five patients with dyspnea in this study had type I respiratory failure and were treated in the ICU. The median incubation period for COVID-19 in our patients was six days; while it was four and eight days in Jiangsu Province and Wuhan city, respectively, which may be related to the disease evolution and the measures of prevention and control in different places. In this study, the incubation period of patients treated in the ICU was similar to that of non-ICU patients. However, the period from symptom onset to hospital admission was 4.5 days longer in ICU patients than in non-ICU patients, suggesting that the delay in treatment may be one of the reasons for the worsening of the disease condition. In addition, patients treated in the ICU were 13 years older than the non-ICU patients and were accompanied by more than two underlying diseases, suggesting that age and underlying diseases of the patients may be the causes of severe illness. Therefore, there were some differences in epidemiology and clinical symptoms between COVID-19 patients in Anhui Province, Wuhan, and Jiangsu Province (Table 5).
Lymphocytes and CD4/CD8 are sensitive indicators for evaluating the immune function of the body. In this study, lymphopenia occurred in 46% of hospitalized COVID-19 patients and was common in the patients treated in ICU (61%). The proportion of COVID-19 patients with lymphopenia in Wuhan was 63%. Fifty percent of the patients had their CD4/CD8 ratio lower than 1.1. In addition, inflammatory indicators such as ESR, CRP, and IL-6 were significantly higher in the ICU patients than in non- ICU patients, suggesting that the patients’ body suffered immune damage in the early course of the disease and was at the peak of the inflammatory response [9].
Among the COVID-19 patients in this study, 92% had their chest images showing inflammatory changes in the peripheral lung regions, 56% had typical ground-glass opacities, and 36% had bilateral diffuse ground-glass opacities accompanied by partial pulmonary consolidation. Therefore, due to the limitation of viral nucleic acid testing conditions, pulmonary CT scan can be used as a preliminary screening strategy for patients at the early stage of the disease. However, six COVID-19 patients had negative findings in pulmonary CT at hospital admission. In addition, one confirmed COVID-19 case in this study showed substantial ground-glass opacities in the pulmonary CT. Interestingly, this patient had four consecutive negative results of viral nucleic acid testing of throat swabs before hospital admission. Therefore, epidemiological history, clinical symptoms, and imaging findings should be comprehensively analyzed for the diagnosis of COVID-19.
With the in-depth study of COVID-19, the various results have also guided clinicians to continuously improve the treatment plan, and individualized treatment based on a patient’s conditions is still the current consensus. Of our 73 COVID-19 patients who were cured and discharged, except for one pediatric and one death cases, the rest of the patients were given oral antiviral therapy with lopinavir and ritonavir tablets. After determining whether the patients were accompanied by or had a lung infection, 81% of the COVID-19 patients were given antibiotic prophylaxis and treatment. Steroid pulse therapy was given to severe patients (approximately 5%) who showed rapid pulmonary imaging progression, and this was lower than those reported in Wuhan, Zhejiang Province, and the whole country. Seventy-one percent of hospitalized COVID-19 patients in this study required oxygen therapy, and 83% of the ICU patients were treated with high-flow oxygen therapy. No invasive ventilation was used for the patients in this study. Four patients with extensive involvement of both lungs and high levels of IL-6 were treated with the immunosuppressive drug tocilizumab. These patients were enrolled in a registered clinical trial, and the results of the trial are still unknown. The median hospital stay of 73 cured patients was 13 days, which was similar to the results reported by Guan et al. (12 days) [10], suggesting that the time window for the immune system to recover in COVID-19 patients was approximately two weeks.
All patients in this study underwent approximately six viral nucleic acid tests (including two negative results). When the clinical signs and imaging results of the patients improved, the viral nucleic acids in the body was likely still in the eradication stage. A study reported that four patients with COVID-19 who met the discharge or quarantine criteria were all positive for the viral nucleic acid testing after 5 to 13 days, suggesting that at least some of the patients who recovered from COVID-19 may still be carriers of the virus [11]. Out study showed that one of the COVID-19 patients had a transient positive result of the viral nucleic acid re-testing in the follow-up period and had no sign of viral transmission, suggesting that the viral nucleic acid fragments may be discharged instead of the virus particles. However, more case studies are needed to confirm.
COVID-19 may vary in different regions, and COVID-19 patients in cities outside Wuhan were relatively mild. Approximately half of the COVID-19 patients in our hospital had a history of close contact with people from Wuhan and were mainly imported cases. Fever, cough, expectoration, and fatigue were the most common symptoms in our patients. Although greater than one-fifth of the hospitalized COVID-19 patients had more than two underlying diseases, individualized treatments achieved satisfactory clinical results in this study.
Citation: Jiazhao Y (2020) Clinical Characteristics, Treatment, and Prognosis of 74 COVID-19 Patients from Cities Outside Wuhan: A Descriptive Study. J Clin Cell Immunol.11:592. doi: 10.35248/2155-9899.20.11:592.
Received: 09-May-2020 Accepted: 22-May-2020 Published: 29-May-2020 , DOI: 10.35248/2155-9899.20.11.592
Copyright: © 2020 Jiazhao Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.