International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2023)Volume 11, Issue 6
Background: Low-intensity Continuous Ultrasound (LICUS) therapy heals soft tissue injuries. It alleviates acute and chronic musculoskeletal pain by activating multiple healing processes through its diathermic and mechanotransductive properties. Diclofenac has been FDA-approved as a Non-Steroidal Anti-Inflammatory Drug (NSAID). It is an analgesic and anti- inflammatory drug available in oral and topical forms. Adding 2.5% diclofenac sodium to ultrasound coupling gel improves ultrasound penetration deeper into the tissue without altering the diathermic and acoustic properties of LICUS and the analgesic effects of diclofenac sodium with no undesired adverse effects.
Objective: To determine the effects of adding 2.5% diclofenac sodium to standard aqueous ultrasound gel on the ultrasound coupling and diathermic properties of a long duration Sustained Acoustic Medicine (SAM) treatment.
Methods: In a two-phase study, first, the acoustic and diathermic changes were determined in bovine tissue during 4-hour-long SAM stimulation at 1 cm, 2 cm, and 5 cm with aqueous and 2.5% diclofenac ultrasound couple patch. Then, in the second phase, the heating profiles were recorded with and without 2.5% diclofenac gels in 54 healthy adult subjects at the forearm and calf during the SAM treatment.
Result: The addition of 2.5% diclofenac sodium significantly increased coupling gel density, acoustic impedance, and signal propagation (p<0.0001) with little or no effect diathermic profiles at 1 cm, 2 cm, and 5 cm depth. The coupling gel with 2.5% diclofenac sodium sustained the therapeutic ultrasound intensity longer than the aqueous coupling gel (5.5 cm relative to 4.5, p<0.0009). No significant diathermic difference was recorded on the calf and forearm skin with a 2.5% diclofenac ultrasound patch.
Conclusion: Adding 2.5% diclofenac sodium to ultrasound gel increases acoustic impedance, improves ultrasound signal coupling into deep tissue, and provides longer sustained deep tissue heating without negatively impacting the diathermic profile during SAM treatment.
Ultrasound; Diclofenac; Diathermy; Acoustic impedance; Sustained acoustic medicine
Low-Intensity Continuous Ultrasound (LICUS) therapy is increasingly used to heal soft tissue injuries and relieve pain in acute and chronic musculoskeletal ailments [1-4]. Ultrasound penetrates the damaged tissue to enhance the healing process by several mechanisms, including diathermy and mechanotransduction [5- 8]. The localized increase in the temperature, cellular migration, and biomolecular activity enhances circulation, vasodilation, oxygenation, and nutrient exchange, boosting cellular metabolism and tissue regeneration [6-11]. The LICUS is transmitted at frequencies between 1 and 3 MHz at intensities of less than 0.5 W/cm2 for 10 minutes to 4 hours [4,12]. The consistent and soothing vigorous heat penetrates deep into connective tissue and muscles with little to no adverse effects on the tissue [9,13]. The application of low-intensity ultrasound between 2-20 MHz is also used in clinical diagnostic and imaging purposes, depending on the application and target tissue. The diagnostic and imaging use of ultrasound relies on the difference in acoustic impedance of tissues. The use of High-Intensity Focused Ultrasound (HIFUS) uses>10 W/cm2 intensity, generating localized heating, more commonly applied in targeting solid tumors. The HIFUS can be used as an alternative to invasive surgery [14]. Diclofenac Sodium is a broadly used Non-Steroidal Anti-Inflammatory Drug (NSAID). It is an analgesic, anti-inflammatory, and antipyretic agent [15]. It acts by inhibiting cyclooxygenase-1 and 2, regulating the production of prostaglandin and various pro-inflammatory biomarkers, including Tumor Factor alpha (TNFα), Interleukin-6 (IL-6), and Interleukin-8 (IL-8) [16]. Topical diclofenac has been shown to be efficacious in osteoarthritis, rheumatic arthritis, and soft tissue pain injuries such as sprains, ligament partial or total rupture, and contusions [16]. Oral diclofenac has been shown to have adverse vascular and gastrointestinal effects, and the efficacy of topical diclofenac is limited due to limited penetration through the skin tissue [15,17,18]. The ultrasound acoustic waves are generated by a piezoelectric element and require a dense medium to travel. Viscous ultrasound gel facilitates acoustic wave transmission from the transducer to the target tissue. The presence of the dense impedance-matched medium allows the transmission of acoustic vibration to the tissue with little or no loss of acoustic energy and intensity. In addition, the localized acoustic force can provide essential mechanical and thermal stimulation to increase profusion, porosity, and cellular activity [19,20], thereby synchronizing ultrasound’s mechano-transduction and diathermic properties along with topical NSAID application benefits [19,21,22]. The United States Food and Drug Administration (FDA) has cleared Sustained Acoustic Medicine (SAM) for treating musculoskeletal injuries. SAM provides low-intensity continuous ultrasound at 1.3 W and 0.132 W/cm2, which equates to about 5,000 Joules per hour or just under 20,000 Joules for a typical 4-hour treatment session [10,21,23]. The meta-analysis by Winkler, et al. demonstrated the clinical efficacy of the SAM in numerous musculoskeletal disorders, including myofascial pain, rotator cuff tendinopathy, knee osteoarthritis, and upper shoulder and neck pain [23]. In addition, Uddin, et al. have elaborated on the underlying mechanism of LICUS and SAM in multiple pathologies such as traumatic bone fracture, osteoarthritis, thrombosis, and pain management [6].
The use of SAM with diclofenac ultrasound gel has been reported by Madzia et al. The SAM treatment with 1% topical diclofenac significantly alleviated the osteoarthritis-induced pain and improved Western Ontario McMaster Outcome (WOMAC) after 7 days of treatment [24]. The treatment efficacy and improved outcomes were attributed to the diathermic and mechanotransducive abilities of the SAM treatment over a 4-hour time. Jarit, et al. presented the use of SAM with 2.5% diclofenac in the treatment of musculoskeletal injuries that failed to respond to physical therapy [25]. After 4-weeks of daily home use, patients reported a significant pain reduction, global health, and functional improvement with SAM treatment. This study aims to determine the ultrasound coupling and diathermic effects of adding 2.5% diclofenac sodium to the ultrasound coupling patch in the SAM treatment system.
Instrumentation
The FDA-cleared SAM device (model sam-12, ZetrOZ Systems LLC., Trumbull, CT) consists of a pair of small rechargeable wireless transducer heads, clipping onto an adhesive patch preloaded with either standard coupling ultrasound gel or 2.5 w/v diclofenac coupling patch. Adhesive patches are directly applied to the skin above the treatment area. The SAM device provides continuous low-intensity continuous ultrasound at 0.132 W/cm2 for a 4-hour treatment delivering 18,720 joules at 3MHz with 0.65W of power from a 5 cm2 radius transducer head.
Laboratory methods
Intensity measurement: The therapeutic intensity in bovine tissue at different depths was measured using a hydrophone with both coupling gels. A3 MHZ, 4-cycle, a sinusoidal signal was generated from a Tektronix function generator (model AFG3021B) and ran through a SAM piezoelectric transducer creating a simulated ultrasound signal similar to the sam-12 device. An ONDA Hydrophone (model HNR-1000) was used to measure and record the signal output at different depth levels using a Tektronix oscilloscope (model TDS2014B).
The bovine tissue was sliced into 1 cm, 2 cm, 3 cm, and 4 cm, and 5 cm thickness, 5 cm diameter discs, 6 per measurement. The bovine tissue was placed between an active transducer and the hydrophone and coupled to both pieces using coupling ultrasound gel. The signal was recorded via the hydrophone and input to an oscilloscope, where the intensity was displayed and recorded. The intensity measurements were recorded with aqueous coupling gel and with 2.5% diclofenac coupling gel. The data was displayed on a graph found in the results section below. The effect of 2.5% diclofenac on the speed of sound and acoustic impedance was also quantified using a similar experimental setup without the bovine tissue, as shown in Figures 1A and 1B. The hydrophone was held at a stable, constant distance from the piezo transducer face outputting a consistent ultrasound signal by a preprogramed oscilloscope. Ultrasound gel was inserted between the transducer face and the hydrophone tip. The signal was generated and recorded by the same oscilloscope at two different independent channels concurrently, and the time delay was recorded. The speed of sound was calculated using the Equation below using the experimental setup in Figures 1A and 1B (Figures 1A and 1B).
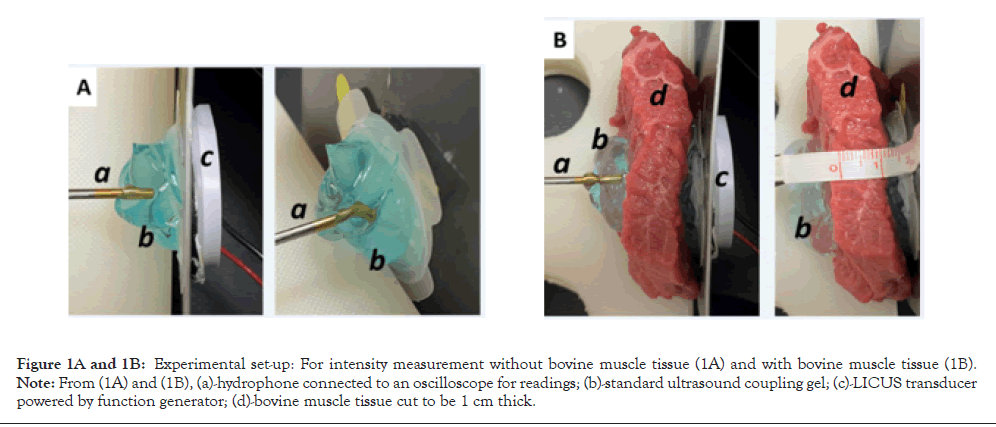
Figure 1A and 1B: Experimental set-up: For intensity measurement without bovine muscle tissue (1A) and with bovine muscle tissue (1B). Note: From (1A) and (1B), (a)-hydrophone connected to an oscilloscope for readings; (b)-standard ultrasound coupling gel; (c)-LICUS transducer powered by function generator; (d)-bovine muscle tissue cut to be 1 cm thick.

speed of sound=(distance (meters))/( time delay (seconds) )
The speed of sound was used to calculate the acoustic impedance based on density determined with a mass and volume calculation using Equation (2). The acoustic impedance was found for both coupling gels, with and without 2.5% diclofenac gel.

Bovine diathermy: Bovine muscle tissue, 800 mg ± 200 mg, bought from a local butcher, was normalized to room temperature and pressure. The ultrasound coupling gel (3 ml) with and without 2.5% diclofenac was placed on the surface of the tissue and well-secured using bandages to ensure to stop any leakage of the gel. The SAM transducer was placed on top of the coupling cup and sealed airtight bandage (Figure 1C). Stainless steel thermocouple probes were stereotactically inserted 2.5 inches into the bovine muscle tissue directly under the center of the transducer. Three probes were stereotactically inserted at 1 cm, 2 cm, and 5 cm depths (Figure 1D). The internal temperatures were continuously monitored during the treatment using OMEGA thermocouple probes (TJ36-ICSS-116G-6-GG) and recorded on a DAQPRO Data logger (OM-DAQPRO-5300). The data logger was set to continuously collect thermocouple K values every ten seconds until the logger was stopped. The tissue was stimulated for 4 hours by the SAM device (Figure 1C and 1D).
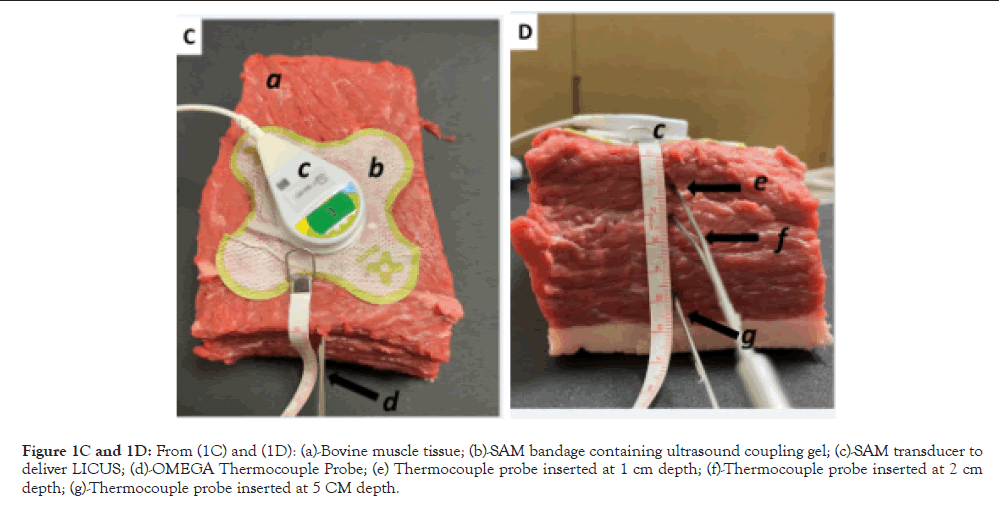
Figure 1C and 1D: From (1C) and (1D): (a)-Bovine muscle tissue; (b)-SAM bandage containing ultrasound coupling gel; (c)-SAM transducer to deliver LICUS; (d)-OMEGA Thermocouple Probe; (e) Thermocouple probe inserted at 1 cm depth; (f)-Thermocouple probe inserted at 2 cm depth; (g)-Thermocouple probe inserted at 5 CM depth.
Clinical methods
Participants: Fifty-six individuals were recruited, and fifty-four healthy male and female subjects between the ages of years 18- 70 were enrolled for this study, with IRB being approved by the Yale New Haven Healthcare System of Eastern Connecticut. The study followed the deceleration of the Declaration of Helsinki and was registered on clinicaltrails.gov (NCT05259995). All subjects signed complete informed consent forms before the start of the study. Pregnant/nursing or subjects with a topical wound in the treatment area were excluded from the study. Subjects were given $100 completion per session. Twenty-six subjects were male, and twenty-eight females between 18-65, averaging 28 years old with 16.4 to 37.3 BMI with an average BMI of 25.8, were selected for the study.
Procedure
Participants were randomly assigned to one of four groups using random number allocation on Microsoft Excel. The study PRISMA flow diagram is shown in Figure 2. Group 1 subjects received SAM ultrasound treatment on the forearm with standard ultrasound coupling gel. Group 2 received the same treatment and standard gel on the calf muscle. Group 3 participants were treated with SAM+2.5% diclofenac ultrasound coupling gel on the forearm. Group 4 was treated with SAM+2.5% diclofenac ultrasound coupling gel on the calf muscle. Figures 3A and 3B show the transducer positioning on the Calf and forearm, respectively (Figure 3A and 3B). All the stimulation sessions were comprehensively completed at the research study site with federalwide assurance. Local temperature readings were recorded for a 4-hour SAM treatment protocol in healthy human volunteers measured with a micro thermocouple probe on the skin surface under the active transducer. The temperature change was recorded at the untreated site as a reference point. Standard aqueous ultrasound coupling gel was used with the transducers in 28 subjects (n=28), and 26 volunteers received treatment using the 2.5% diclofenac ultrasound coupling gel (n=26). Temperatures were recorded at the skin’s surface beneath the active transducer on either the forearm (n=27) or calf muscle (n=27). Control temperatures were recorded at the skin’s surface 6 cm away from the treatment site, as shown in Figures 4A and 4B. Temperatures were measured using an OMEGA micro thermocouple (Model: 5SC-TT-K-40-36) and recorded with the OMEGA DAQPROS, also used in laboratory experiments. The thermocouples adhered to the skin with SAM bandage pieces. The data was uploaded to DAQLAB software on the computer to be analysed (Figures 4A and 4B).
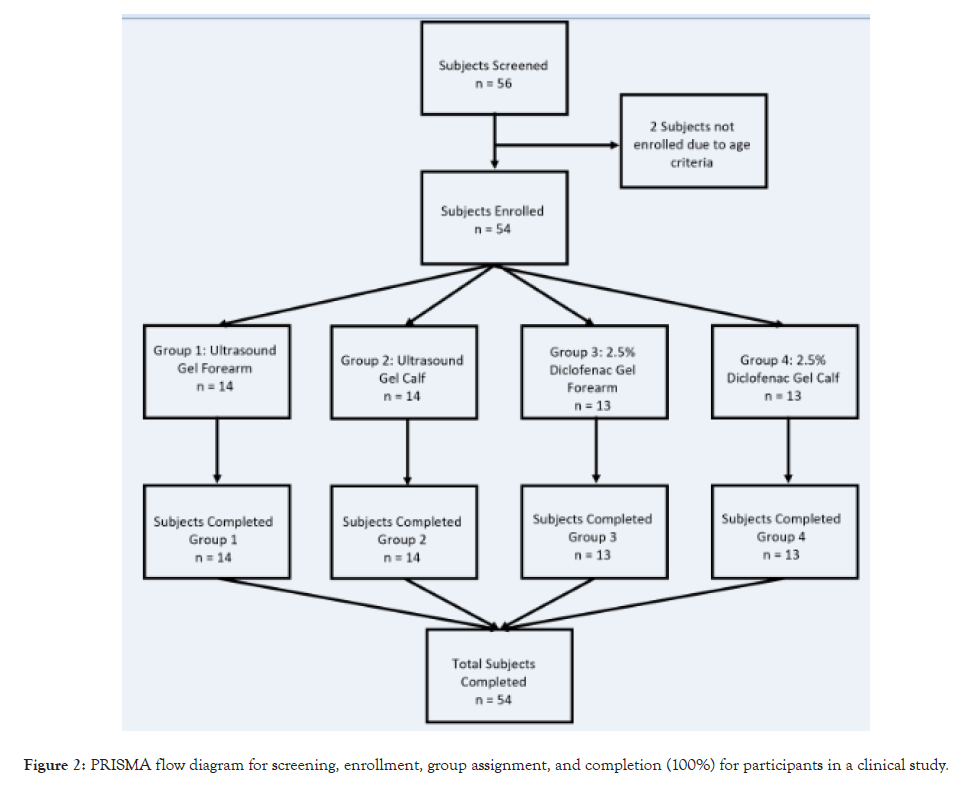
Figure 2: PRISMA flow diagram for screening, enrollment, group assignment, and completion (100%) for participants in a clinical study.
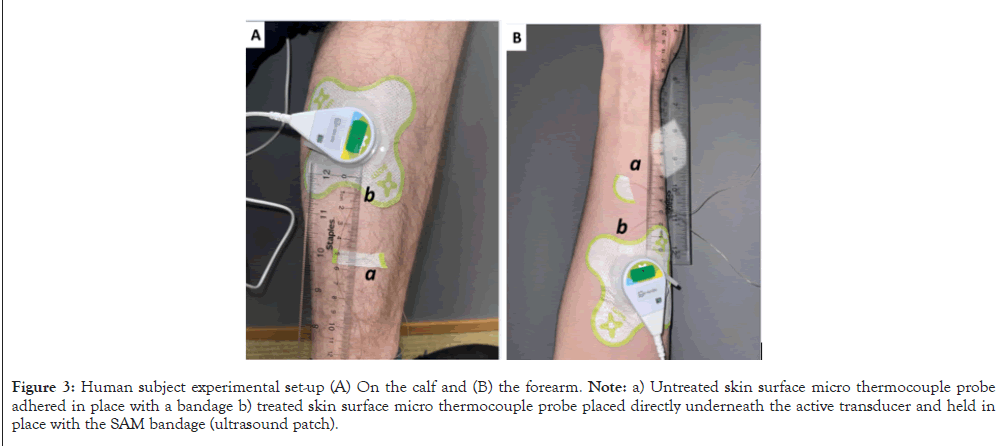
Figure 3: Human subject experimental set-up (A) On the calf and (B) the forearm. Note: a) Untreated skin surface micro thermocouple probe adhered in place with a bandage b) treated skin surface micro thermocouple probe placed directly underneath the active transducer and held in place with the SAM bandage (ultrasound patch).
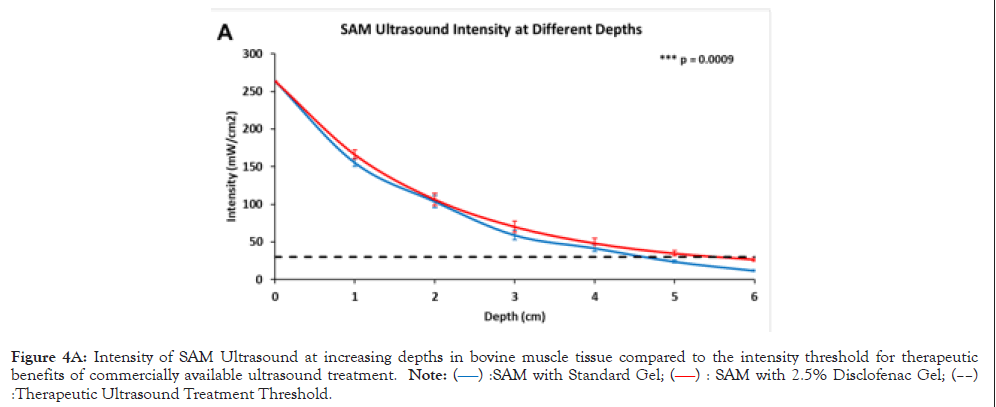
Figure 4A: Intensity of SAM Ultrasound at increasing depths in bovine muscle tissue compared to the intensity threshold for therapeutic benefits of commercially available ultrasound treatment. Note: (â??â?? ):SAM with Standard Gel; (â??â?? ): SAM with 2.5% Disclofenac Gel; (â??â??) :Therapeutic Ultrasound Treatment Threshold.
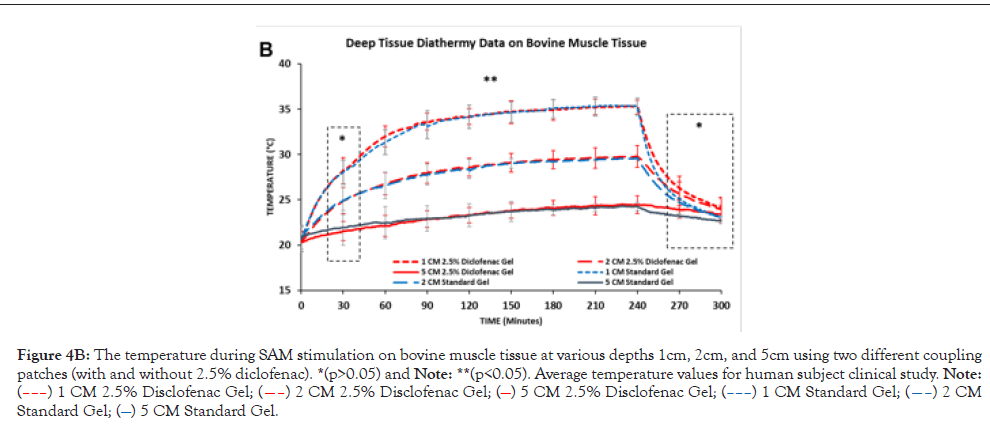
Figure 4B: The temperature during SAM stimulation on bovine muscle tissue at various depths 1cm, 2cm, and 5cm using two different coupling patches (with and without 2.5% diclofenac). *(p>0.05) and Note: **(p<0.05). Average temperature values for human subject clinical study. Note: (–––) 1 CM 2.5% Disclofenac Gel; (––) 2 CM 2.5% Disclofenac Gel; (—) 5 CM 2.5% Disclofenac Gel; (–––) 1 CM Standard Gel; (––) 2 CM Standard Gel; (—) 5 CM Standard Gel.
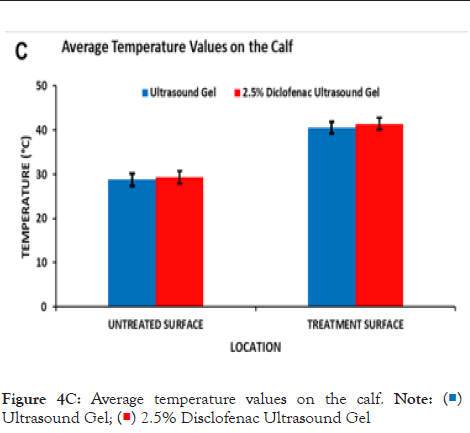
Figure 4C: Average temperature values on the calf. Note: ( )Ultrasound Gel; (
)Ultrasound Gel; ( ) 2.5% Disclofenac Ultrasound Gel
) 2.5% Disclofenac Ultrasound Gel
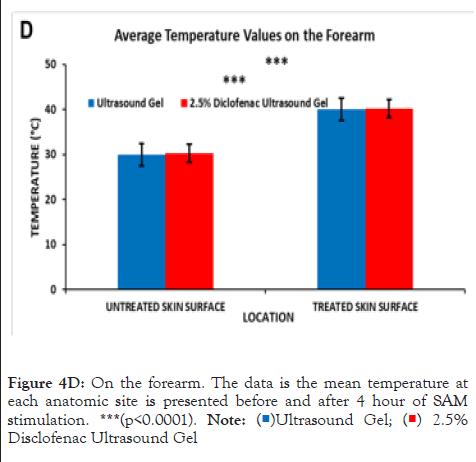
Figure 4D: On the forearm. The data is the mean temperature at
each anatomic site is presented before and after 4 hour of SAM
stimulation. ***(p<0.0001). Note:( )Ultrasound Gel; (
)Ultrasound Gel; ( ) 2.5%
Disclofenac Ultrasound Gel.
) 2.5%
Disclofenac Ultrasound Gel.
Statistical analysis
A statistical analysis was conducted to assess the statistical differences in ultrasonic signal penetration, gel acoustic properties, and temperature performance for the two coupling gels used. T-tests were conducted using sample size, mean with standard deviation and statistical differences of more the p<0.05 were considered significant differences between measurement groups. In addition, the statistical difference between different temperature depths at the same site was evaluated using One-Way ANOVA with Prism GraphPad.
Laboratory results
2.5% Diclofenac gel properties: The speed of sound calculated for 2.5% diclofenac ultrasound gel was 1513.24 ± 2.24 m/s. The speed of sound calculated for the current SAM ultrasound gel was 1512.64 ± 2.39 m/s with no significant difference (p=0.69). Acoustic impedance was calculated using the speed of sound and the density of both gels, respectively. Acoustic impedance for 2.5% diclofenac ultrasound gel was 1.599 ± 0.0001 MPa⸱s/m3 and 1.543 ± 0.002 MPa⸱s/m3 for the SAM ultrasound gel. The acoustic impedance between both coupling gels was statistically different (p<0.0001) (Table 1).
| Standard ultrasound gel | 2.5% Diclofenac gel | p-value (n=5) | |
|---|---|---|---|
| Density (g/mL) | 1.02 | 1.06 | 0.0001 |
| Speed of Sound (m/s) | 1512.64 ± 2.39 | 1513.24 ± 2.24 | 0.6944 |
| Acoustic Impedance (Mpa.s/m3) | 1.54 ± 0.002 | 1.60 ± 0.002 | 0.0001 |
Table 1: Calculated values for Speed of Sound and Acoustic Impedance using equations (1) and (2), given density, and data values collected during intensity measurements (n=5).
Bovine performance testing
The SAM device did not fall below the therapeutic ultrasound threshold of bone-growth stimulators (30 mW/cm2) until approximately 4.5 cm in bovine tissue depth and 5.5 cm depth with 2.5% diclofenac ultrasound gel coupling. The calculated Intensity values confirm that the 30 mW/cm2 threshold passed between 4.5 cm and 5.5 cm, and the addition of 2.5% diclofenac significantly (p=0.009) enhances ultrasound propagation into tissue by approximately 1 cm. The highest acoustic intensity was recorded at the surface of the transducer, and it decayed across the depth. The exponential decay rate was delayed with 2.5% diclofenac, showing improved acoustic impedance matching with the 2.5% diclofenac sodium gel (Table 2).
| Depth (cm) | Intensity with standard gel (mW/cm2) | Intensity with 2.5% diclofenac gel (mW/cm2) |
|---|---|---|
| 0 | 264.00 ± 1.12 | 264.00 ± 1.34 |
| 1 | 155.33 ± 5.37 | 166.04 ± 3.47 |
| 2 | 103.75 ± 7.92 | 106.50 ± 6.94 |
| 3 | 59.12 ± 6.33 | 70.32 ± 5.83 |
| 4 | 41.85 ± 4.51 | 48.33 ± 4.76 |
| 5 | 23.93 ± 1.87 | 34.97 ± 2.22 |
| 6 | 11.97 ± 1.32 | 26.84 ± 1.15 |
Table 2: Ultrasound intensity at different depths stimulated with standard and 2.5% diclofenac ultrasound patches.
The diathermic analysis at 1 cm, 2 cm, and 5 cm in the bovine tissue showed a distinct diathermic profile at each intramuscular depth and distance from the transducer surface. The highest temperature increase and change rate was recorded close to the transducer surface, 1 cm intramuscular depth. The temperature increased from an average of 20.63°C and peaked at approximately 35.31°C after 240 min of the treatment at 1 cm depth, Δ14.68°C was the highest change in tissue temperature for the first 90 min. The smallest temperature increase was recorded at 5 cm intramuscular depth from 20.36°C to 24.56°C with a total increase of Δ4.20°C and gradually increasing over 240 mins. The temperature significantly increased over 240 min (4 hours stimulation). After the treatment, the temperature decrease time was less. At the end of the SAM treatment, temperature reduced significantly over the first 60 mins, with a relatively higher rate of decrease recorded in the bovine tissue treated with 2.5% diclofenac gel due to the deeper therapeutic acoustic penetration. However, no distinctive diathermic profile differences were recorded between the SAM coupling patches without and with 2.5% diclofenac.
Clinical human subject’s results
The effect of the diclofenac coupling patch on skin temperature was assessed in the adult human calf and forearm. Prior to the start of the treatment, skin temperature was 28.78°C ± 1.39°C and 29.29°C ± 1.39°C respectively, without and with 2.5% diclofenac coupling gel at the Calf and 29.96°C ± 1.69°C and 30.32°C ± 1.64°C respectively without and with 2.5% diclofenac coupling gel on the human forearm. After the 4 hours of the SAM treatment, approximately the same increase in the temperature was recorded at both sites (Figures 4C and 4D). The skin temperature at the calf increased to 40.56°C ± 1.33°C and 41.46°C ± 1.73°C respectively, without and with 2.5% diclofenac coupling gel. On the forearm, skin temperature increased to 40.04°C ± 2.17°C and 40.22°C ± 1.95°C respectively, without and with 2.5% diclofenac coupling gel. The presence of diclofenac did not affect the surface temperature change after the 4-hour treatment. No significant effect was recorded at either site before or after treatment due to the presence of 2.5% diclofenac sodium.
Ultrasound is an acoustic energy source generated from the vibration of piezoelectric element-initiated sound waves that requires an aqueous medium to travel from one point to another without dissipating significant energy over a long distance [26-28]. The density of the medium plays an essential role in the travel of ultrasound and associated energy. Thus, it is crucial to understand the impact of a change in density on the thermodynamics of ultrasound and its potential impact on the therapeutic effects. The coupling medium must be liquid enough to flow to fill gaps between the vibrating source (piezoelectric element) and the effector (Human skin, organ). Diclofenac is a commonly used NSAID in musculoskeletal pain management. It has relatively high COX-2 isoenzymes inhibition relative to COX-1 and PGE2 production via monocytes and platelets [15,16,18]. The inhibition of the PGE2 reduces the localized inflammation and pain sensation systematically [29,30]. However, at a lower level, diclofenac also inhibits the COX-1 isoenzymes, which regulate the prostaglandins that protect gastrointestinal mucosa. Thus, the prolonged systemic use of diclofenac disturbs the mucosal lining and results in ulcers and other intestinal and urinary tract complications. The localized, topical application of diclofenac has shown to be effective. However, it is limited due to the limited penetration through the skin tissue, which significantly varies across different skin sites depending on the skin’s depth and porosity. Previous studies have shown that topical application of diclofenac in conjunction with ultrasound stimulation increases the efficacy of treatment. vân Der Biji, et al. has shown that sonification has increased the diclofenac flux rate through human skin specimens [31]. Masterson, et al. showed increased NSAID penetration through skin-like tissues by 3.8 fold when actively stimulated by the SAM device for 4 hours [21]. Bukhari, et al. has shown an improved reduction in swelling after acute injuries at different anatomical sites in human subject’s post-diclofenac sonophoretic treatment [19]. In a multi-center study, Madzia, et al. have shown alleviated pain reduction and improvement in WOMAC score in knee osteoarthritis patients after being treated with SAM with 1% diclofenac hydrogel for 7 days [24]. Jarit, et al. has shown significant injury pain relief in tendon, muscle, and bone injuries with SAM with 2.5% diclofenac gel for 4 weeks [25]. Multiple studies have shown synergistic responses of ultrasound and diclofenac application [19,21,24,32]. This study for time shows that the addition of diclofenac can potentially enhance the ultrasound acoustic penetration into the biological tissue. The long-duration SAM treatment renders it essential to ensure that additional 2.5% diclofenac sodium would not negatively impact the diathermic properties of SAM treatment and potentially cause undesired adverse effects. The data from this study show that the addition of 2.5% diclofenac to the gel changes the density of the coupling patch and improves ultrasound coupling into deeper tissue. The diclofenac gel coupling provides similar diathermic heating properties through the 5 cm of the bovine tissue while providing slower cooling and deeper ultrasonic signal propagation. The slower cooling directly results from an overall increase in ultrasound transmission into the bovine muscle tissue (more energy delivered) due to the improved acoustic impedance characteristics of the 2.5% diclofenac ultrasound gel.
This can also be visualized with generally higher diathermy measurements at 2 cm and 5 cm across all time points measured, including the cool-down phase. This study, for the first time, shows adding 2.5% diclofenac sodium improves ultrasound coupling into deep soft tissue and can be potentially used for long-duration SAM treatment in therapeutic applications. The study has some limitations as it uses bovine tissue for deep tissue measurements; future studies may use ex vivo human tissue specimens or large porcine animal models to further assess the efficacy of the long-term SAM treatment with 2.5% diclofenac.
The authors would like to thank the National Institute of Health (NIH) for the support and funding of this research Grant R44AG061985.
TH. NY and KB all contributed to the research of this manuscript. In addition, TH and NY contributed to the data analysis of this manuscript, and TH conducted the writing of this manuscript.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Hendren TF, Yeretzian NR, Bavanasi K (2023) Clinical Diathermy Performance Evaluation of Multi-hour Sustained Acoustic Medicine Treatment with 2.5% Diclofenac Ultrasound Coupling Patch. Int J Phys Med Rehabil. 11:678
Received: 17-May-2023, Manuscript No. JPMR-23-24203; Editor assigned: 19-May-2023, Pre QC No. JPMR-23-24203 (PQ); Reviewed: 06-Jun-2023, QC No. JPMR-23-24203; Revised: 13-Jun-2023, Manuscript No. JPMR-23-24203 (R); Published: 20-Jun-2023 , DOI: 10.35248/2329-9096.23.11.678
Copyright: © 2023 Hendren TF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited